Originally published : Fri, October 14, 2022 @ 2:50 PM
Updated : Tue, May 9, 2023 @ 11:03 AM
When it comes to oligonucleotide therapeutics, the challenge has always been how to deliver an intact oligo to the right cells. Now, with sixteen oligonucleotide therapeutics approved globally as of December 2021, and eleven of these approved since 2016,1 it’s clear that robust solutions are here.
One delivery approach that the team at LGC Biosearch Technologies is very excited about is N-acetylgalactosamine (GalNAc), which provides highly specific targeting to the liver and is used in four of the five approved siRNA therapeutics: Lumasiran, Givosiran, Vutrisiran and Inclisiran. These relatively recent drug approvals demonstrate that delivery of oligonucleotides with GalNAc has reached clinical success, creating a large field of opportunity for discovery and application in the liver target space.
In this article we’ll talk about why we are so excited about GalNAc for delivering siRNAs and other therapeutic oligos to the liver and how we think that GalNAc is ushering in a new generation of oligo therapeutics.
GalNAc enables access to a wide-open target space in the liver
|
Once believed by the ancient Greeks to be the seat of the human soul,2 the liver is the second largest organ of the body and, when dysfunctional, a leading cause of death worldwide.3 While many liver-related diseases can be prevented through lifestyle changes, the burden of disease remains a global challenge.3 Therapeutic oligonucleotides could be a response to this large unmet need. With an estimated ~17,000 genes expressed in the liver,4 less than 1% of which have been targeted by publicly-known siRNA sequences (figure 1), the potential drug target space in the liver is very large. GalNAc technology can enable access to this large target space. |
Figure 1. Less than 1% of the estimated 17,000 genes expressed in the liver have been targeted by publicly-known siRNA sequences. |
GalNAc provides a simple and defined system for targeting the liver
While the first approved siRNA therapeutic, Patisiran, used a lipid nanoparticle delivery (LNP) system, the other four have used GalNAc. GalNAc is a highly specific ligand for the asialoglycoprotein receptor (ASGPR), which is predominantly expressed on the surface of hepatocytes.5 As Springer and Dowdy write, “Unlike complicated LNP formulations, GalNAc-siRNA conjugates are a simpler, smaller, and represent a compositionally defined approach for hepatic delivery.”5
Compared to free antisense oligonucleotides, GalNAc conjugation enhances hepatocyte delivery ~10-fold in preclinical models, enabling lower dosing.6 Cell entry is believed to occur during internalisation and recycling of ASPGR (figure 2). The GalNAc-siRNA-ASPGR complex is engulfed into an endosome where the low pH induces the release of the GalNAc-siRNA from the ASPGR. Endosomal glycosidases cleave the siRNA from the GalNAc, and free siRNA escapes from the endosome through an unknown mechanism.5 Once inside the cell, the free siRNA can engage with the target and induce an RNAi response.
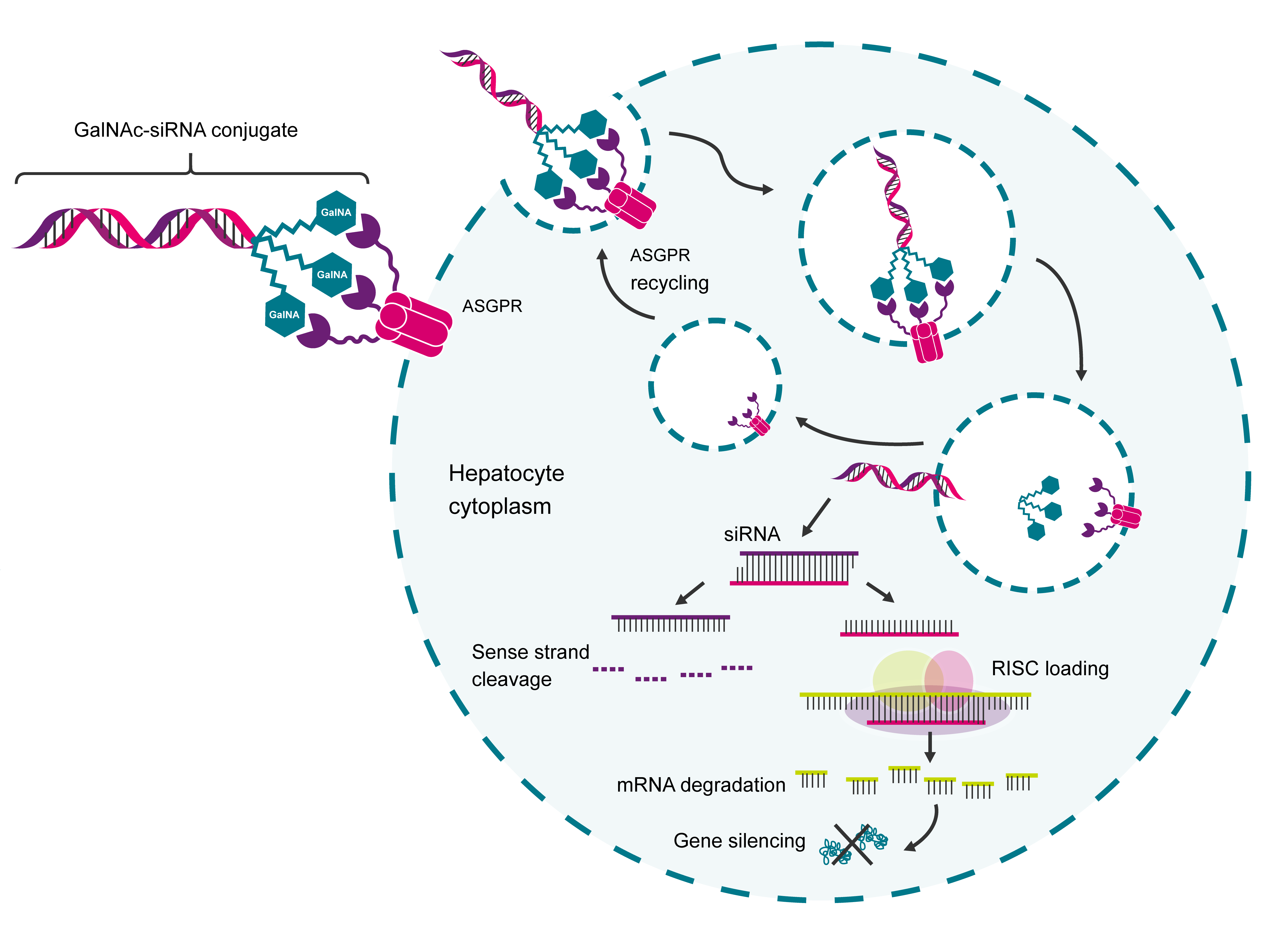
|
|
Figure 2. GalNAc-siRNA conjugates are believed to enter the cell via endocytosis of the GalNAc-siRNA-ASPGR complex. |
Optimisation of oligonucleotide chemistry has been the key enabler for GalNAc
The key to successful implementation of GalNAc for siRNA delivery is extensive optimisation of the chemistry of the conjugate.7 By optimising the chemistry at the 2′ position of the nucleotides and replacing phosphodiester bonds with phosphorothioate bonds, the GalNAc-oligo conjugate becomes stable enough to reach the target cells after intravenous or subcutaneous administration.7
Looking forward to more targeted oligonucleotide therapeutics in the future
With an ability to successfully target siRNA delivery to hepatocytes, straightforward and well-defined chemistry, and a wide-open target space, GalNAc has opened the door to a myriad of possibilities for liver-targeted siRNA and antisense oligonucleotide therapeutics. We are looking forward to seeing how discovery and development teams make use of this promising technology and are excited to support your efforts targeting the liver and other tissues with custom oligo synthesis, including highly modified oligos and GalNAc conjugates, as well as GalNAc phosphoramidites and CPG solid supports.
Find out how you can accelerate the development of your liver-targeted therapeutics with LGC.
Related content from our blog:
- Nucleic acid therapeutics for next-generation disease management
- Overcoming manufacturing challenges associated with nucleic acid therapeutics
References
- Igarashi J, Niwa Y, Sugiyama D. Research and development of oligonucleotide therapeutics in Japan for rare diseases. Future Rare Dis. 2022;2(1):FRD19. doi:10.2217/frd-2021-0008
- Tiniakos DG, Kandilis A, Geller SA. Tityus: A forgotten myth of liver regeneration. J Hepatol. 2010;53(2):357-361. doi:10.1016/j.jhep.2010.02.032
- Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151-171. doi:10.1016/j.jhep.2018.09.014
- Yu Y, Ping J, Chen H, et al. A comparative analysis of liver transcriptome suggests divergent liver function among human, mouse and rat. Genomics. 2010;96(5):281-289. doi:10.1016/j.ygeno.2010.08.003
- Springer AD, Dowdy SF. GalNAc-siRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics. Nucleic Acid Ther. 2018;28(3):109-118. doi:10.1089/nat.2018.0736
- Prakash TP, Graham MJ, Yu J, et al. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 2014;42(13):8796-8807. doi:10.1093/nar/gku531
- Debacker AJ, Voutila J, Catley M, Blakey D, Habib N. Delivery of Oligonucleotides to the Liver with GalNAc: From Research to Registered Therapeutic Drug. Mol Ther. 2020;28(8):1759-1771. doi:10.1016/j.ymthe.2020.06.015


