Originally published : Wed, February 23, 2022 @ 9:40 PM
Updated : Tue, March 21, 2023 @ 3:44 PM
This is the first blog post in a two-part series exploring how oligonucleotide modifications can support the advancement of nucleic acid therapeutics. The second blog post covers the technologies and chemical modifications available that can be harnessed to use nucleic acids for therapeutic applications.
Researchers have studied nucleic acid therapies for more than two decades, but they’ve recently gained more attention as mRNA vaccines for COVID-19 made their clinical debut. Although nucleic acid therapeutics technology shows promise for treating unmanageable diseases using current drug therapies, some challenges stand in the way of broader application.
What are nucleic acid therapeutics?
After years of research, nucleic acids are now recognised as the third major drug discovery platform in addition to small molecules and antibodies.1 Nucleic acid therapeutics use synthetic oligonucleotides to achieve long-lasting or curative effects through gene inhibition, addition, replacement or editing.2 In essence, a gene’s expression can be manipulated to achieve the desired therapeutic effect - whether that’s turning off protein production or stimulating it. This genetic modulation is a different mode of action than conventional drugs, which generally target proteins to induce therapeutic effects.2
Four platform technologies - chemically modified antisense oligonucleotides (ASOs), N-acetylgalactosamine (GalNAc) ligand-modified short interfering RNA (siRNA) conjugates, lipid nanoparticles (LNPs) and adeno-associated virus (AAV) vectors - support the vast majority of approved or late-stage clinical nucleic acid therapeutic candidates.2 ASOs and siRNAs induce therapeutic effects by disrupting the transcription of nuclear DNA into mRNA and the translation of mRNA into proteins, which effectively silences genes without modifying them.3 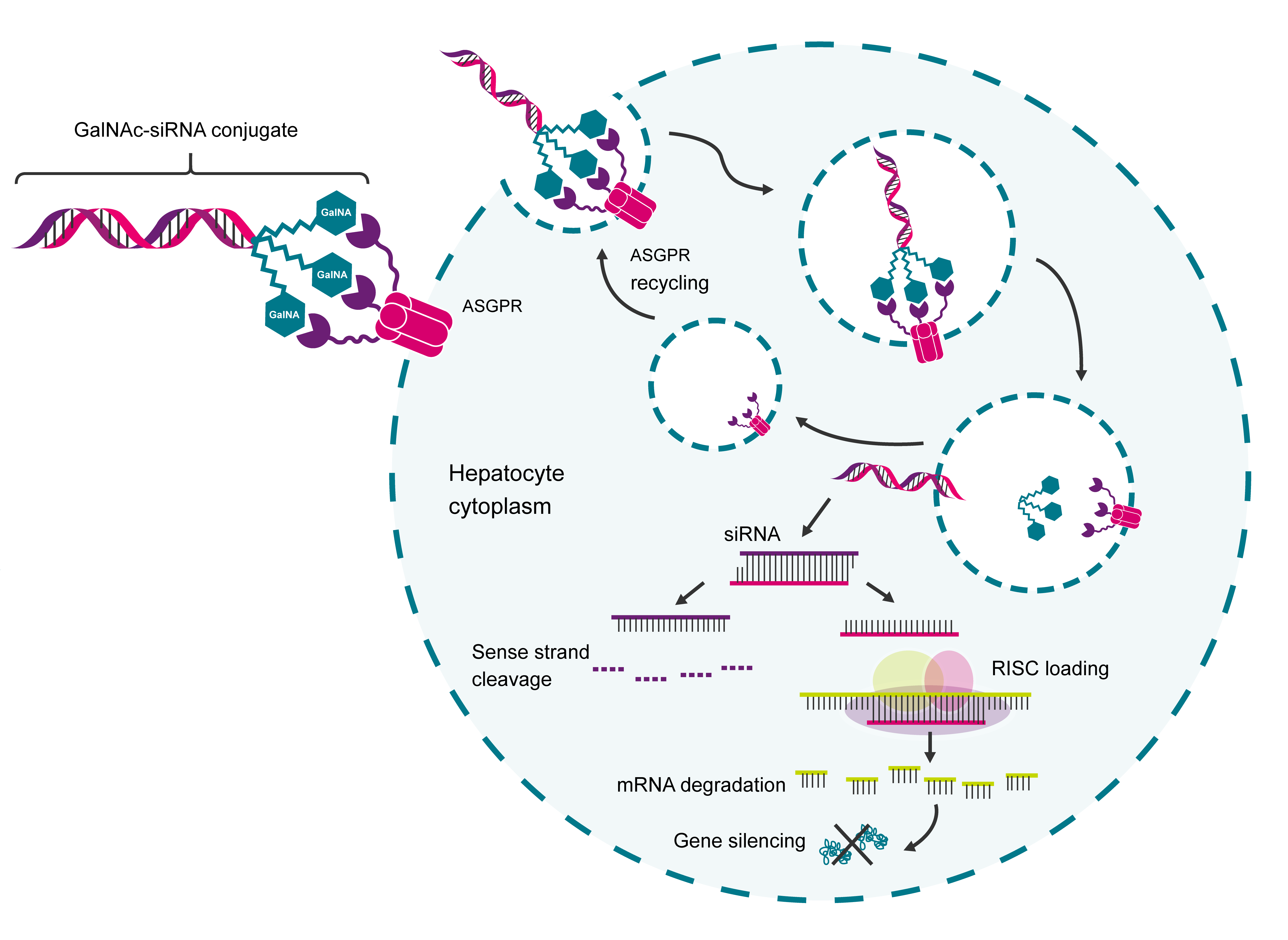
Figure 1. Depiction of GalNAc conjugate delivering RNA-based therapeutics within the liver. GalNAc binds to the highly liver-expressed asialoglycoprotein receptor (ASPGR) with high affinity and specificity, which results in significant hepatocyte uptake. Following internalisation, the conjugate remains in the cell while ASGPR is recycled back to the cell surface, enabling efficient and potent therapeutic delivery to the liver.
While most currently approved nucleic acid therapeutics treat rare diseases, their delivery technologies can be leveraged to explore treatments for more common diseases, including cancer, cardiovascular diseases and haemophilia.4 Even with this promising future, challenges stand in the way of widespread implementation of nucleic acid therapeutics. For more broad clinical application, manufacturing obstacles, toxicity issues and economic barriers must be overcome.2
Manufacturing challenges for nucleic acid therapeutics
Nucleic acids are challenging for therapeutic applications because they are susceptible to nuclease degradation, are difficult to deliver to a cell and contribute to immune response.2 For the effective clinical success of nucleic acid therapeutics, improved technology for the stability, internalisation and target affinity of the synthetic oligonucleotides is required.2
Cellular enzymes pose a significant threat to nucleic acid therapeutics. Endonucleases and exonucleases attack DNA and RNA molecules at the phosphodiester bridges and break them down into mononucleotides, ultimately making unmodified oligonucleotides useless for therapeutic applications.5 To create a functional oligonucleotide suitable for disease treatment, its design must offer sufficient resistance to nuclease degradation in the cell.
Efficient nucleic acid cell delivery remains one of the most critical challenges for researchers to overcome. RNA-based therapeutics are relatively large, highly anionic molecules, making them difficult to diffuse across cellular membranes. They can’t enter cells independently and instead rely on a delivery agent to traverse into a cell. In addition, the oligonucleotide must avoid filtration by the kidneys and uptake by non-targeted cells.
Targeted cell delivery of RNA drugs is required to reduce off-target accumulation in organs and remains a challenge in tissues other than the liver.3 ASOs, in particular, commonly concentrate in the kidneys, liver and spleen, resulting in toxicity. Optimised delivery platforms can help reduce ASO dosage requirements, increase efficiency and allow tissue-specific targeting to minimise their toxicity.6 While several cell delivery platforms are currently in use that can help address these issues, scalability and reproducibility at the industrial scale may limit practical application.6
Enabling therapeutic applications
Fortunately, chemical modifications and technologies are available to address some of the challenges associated with using nucleic acids for therapeutic applications. These modifications can confer nuclease-resistance, improve stability and target-binding affinity and promote more efficient cell delivery and uptake.2
Check out our second blog post of the series which dives deep into the chemical modifications that enable therapeutic applications.
 |
If you are thinking about bringing oligo synthesis in-house, there are many considerations to account for beyond instrument selection. But the benefits of Download this guide that summarises all the practical and technical information in a single place, to ensure that your synthesizers run at full capacity and that you maximise your return on investment. |
References
- Juliano R. The delivery of therapeutic oligonucleotides. Nucleic Acids Research, 44 (14): 6518-6548. Published August 2016. Accessed 01 September 2021. https://doi.org/10.1093/nar/gkw236
- Kulkarni JA, Witzigmann D, Thomson SB, et al. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 16, 630–643. Published May 2021. Accessed 01 September 2021. https://doi.org/10.1038/s41565-021-00898-0
- The expanding frontier of nucleic acid therapeutics. Nature. Accessed 03 September 2021. https://www.nature.com/articles/d42473-021-00174-8
- Peters J. Haven’t heard of RNA therapy yet? You will. Massive Science. Published August 2018. Accessed 03 September 2021. https://massivesci.com/articles/rna-therapy-treatments/
- Gewirtz AM, Sokol DL, Ratajczak MZ. Nucleic acid therapeutics: state of the art and future prospects. Blood. 92 (3): 712–736. Published August 1998. Accessed 01 September 2021. https://doi.org/10.1182/blood.V92.3.712
- Dhuri K, Bechtold C, Quijano E, et al. Antisense oligonucleotides: an emerging area in drug discovery and development. Journal of Clinical Medicine. 9(6) 2004. Published June 2020. Accessed 25 August 2021. https://doi.org/10.3390/jcm9062004


