Originally published : Fri, March 1, 2024 @ 7:38 PM
In this latest instalment of our 'Know Your Oligo Mod' blog series, we explore the benefits of high-performance ATTO fluorescent dyes for labelling oligonucleotides.
ATTO fluorescent dyes are a family of synthetic organic molecules that are widely used as fluorescent labels for biological imaging and analysis. They are developed by ATTO-Tec GmbH, in Germany, and are known for their high photostability and brightness compared to other fluorescent dyes of the same wavelength.
Fluorescent labelling of oligonucleotides has numerous applications as probes and primers in molecular biology techniques, including real-time PCR, DNA sequencing, fluorescence in situ hybridisation (FISH), and gene expression analysis (mRNA analysis). ATTO dyes are particularly notable for their superior performance and versatility. Read on to discover the unique properties of ATTO dyes that make them a perfect fit for labelling oligos.
What sets ATTO dyes apart?
ATTO dyes are characterised by high fluorescence, quantum yields, strong absorption and increased thermal and photo-stability. These beneficial properties are due to their unique molecular structure.
Unlike most common dyes, such as cyanines, which have a flexible structure, ATTO dyes feature a highly rigid chromophore (figure 1). This rigid structure prevents the formation of isomers in solution, ensuring consistent optical properties. While many other dyes are prone to variations in their optical properties, ATTO dyes perform nearly independently of solvent and temperature. Many ATTO dyes also have additional properties, such as a large Stokes-shift, high ozone resistance and good water solubility.1
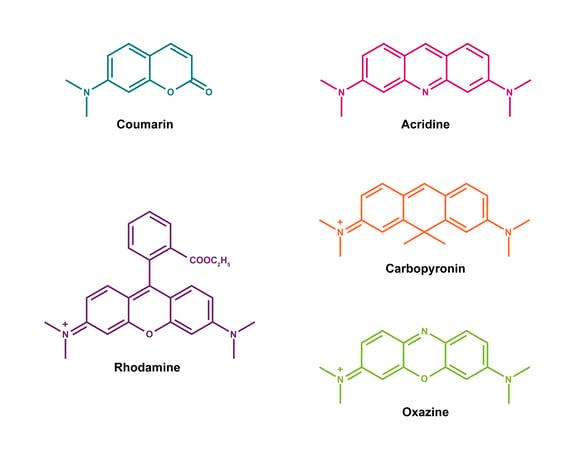
Figure 1: Molecular structure of ATTO dyes. Most ATTO-labels are derivatives of coumarin, acridine, rhodamine, carbopyronin and oxazine.
|
Terminology quick reference Fluorescence quantum yield The efficiency at which a fluorescent molecule converts absorbed photons into emitted photons.4 The brightness of a fluorophore is a measure of the extinction coefficient (the quantity of absorbed light at a given wavelength) and the quantum yield (given as a ratio of emitted: absorbed photons). Therefore a higher fluorescence quantum yield value contributes to a brighter fluorophore. Stokes-shift The difference between the absorption and emission peak for fluorophores. A larger Stokes-shift reduces self-quenching and improves the signal-to-noise ratio. This also minimises the inner-filter effect, and is advantageous in multicolour imaging.5 Photostability Photostability refers to its resistance to damage when exposed to light and reactive species like oxygen. The triplet state of the fluorophore can cause bleaching, therefore molecular modifications to decrease oxidation susceptibility can improve photostability.6 Rayleigh and Raman scattering Described as light's interaction with matter, including biomolecules and fluorophores. Rayleigh scattering causes light to scatter in all directions with no change in wavelength, while Raman scattering causes a change in wavelength. Both can interfere with fluorescence measurements in biological experiments by causing background signal or affecting the excitation and emission of the fluorophore. |
Advantages of ATTO dyes
ATTO dyes cover a broad spectral range – from 350 nm in the UV range to 750 nm in the near infrared – and include dyes compatible with the most frequently used excitation sources. Their broad excitation signal maxima range and good Stokes-shift separation make them suitable for most techniques, while also enabling multiplexing.
Advantages of ATTO dyes include:
-
Reduced background/noise: Many ATTO dyes can be stimulated by wavelengths beyond 600 nm. The use of these long wavelength activated ATTO dyes with the right excitation wavelength can decrease autofluorescence, as well as background fluorescence from Rayleigh and Raman scattering, thereby enhancing sensitivity in biological analysis and imaging techniques.
-
High photostability: ATTO-labels are designed with improved stability, even under prolonged irradiation. ATTO 655 and ATTO 647N are also resistant to ozone degradation. Moreover, most dyes’ hydrophilic nature makes them highly suitable for labelling in aqueous solutions.
-
Improved signal detection: ATTO dyes have longer fluorescence signal lifetimes ranging from 0.6 to 4.1 ns, compared to carbocyanine dyes and many inherent autofluorescent biomolecules. This allows clear and more accurate signal measurement using pulsed laser excitation with a time-gated detection system, resulting in decreased interference from fluorophores with shorter lifetimes, background autofluorescence, as well as Rayleigh and Raman light scattering.
-
Multiplexing ability: ATTO dyes are well-suited for multiplex techniques using visible and near infrared emission wavelengths because of their strong fluorescent signals, with most of them having molar absorptivity values over 100,000. Additionally, their low excitation/emission overlap further enhances their suitability for multiplexing.
ATTO modifications for your oligonucleotides
ATTO dyes are widely used for labelling DNA and RNA oligos, which can be used in diagnostic assays and high-throughput screening applications in drug development. ATTO-dyes in the red spectral region are known to minimise cell damage,2 rendering them suitable for use in live cell experiments, for example, real-time visualisation of mRNA expression.3
LGC Biosearch Technologies offer a range of 5′, internal, and 3′ ATTO modifications for your oligonucleotides. All dyes exhibit high fluorescence yield and can be a good substitute for common fluorescent dyes in experiments where photostability is critical.
Choose from our selection of ATTO labels below to find the perfect fit for your oligos, probes, and primers. Explore our custom oligos ordering page to configure your oligos now.
| Dye | Position | Specs | Features | Applications | Quenchers |
| ATTO 390 | 5′, 3′ | Coumarin derivative |
|
Can be used in a wide array of applications. | BHQ™-1 |
| ATTO 425 | 5′, 3′ | Coumarin derivative |
|
Dual-labelled fluorogenic probes for real-time PCR. | BHQ-0 |
| ATTO 465 | 5′, internal, 3′ | Derived from acriflavine Cationic dye |
|
Dual-labelled fluorogenic probes for real-time PCR. | BHQ-1 |
| ATTO 488 | 5′, 3′ | Rhodamine derivative |
|
Single molecule detection, high-resolution microscopy, and FISH. | BHQ-1 |
| ATTO 490LS | 5′, 3′ |
|
Ideal for applications that require multiplexing. | BHQ-2 | |
| ATTO 514 | 3′ | Rhodamine derivative |
|
Single molecule detection, high-resolution microscopy, and FISH. | BHQ-1 |
| ATTO 520 | 5′, 3′ | Rhodamine derivative |
|
Dual-labelled fluorogenic probes for real-time PCR. | BHQ-1 |
| ATTO 532 | 5′, internal, 3′ | Rhodamine derivative |
|
Single molecule detection, high-resolution microscopy, and FISH. | BHQ-1 |
| ATTO 550 | 5′, 3′ | Rhodamine derivative Cationic dye |
|
Single molecule detection, high-resolution microscopy, and FISH. | BHQ-2 |
| ATTO 565 | 5′, 3′ | Rhodamine derivative |
|
Single molecule detection, high-resolution microscopy, and FISH. | BHQ-2 |
| ATTO rho12 | 5′ | Rhodamine derivative |
|
Dual-labelled fluorogenic probes for real-time PCR | BHQ-2 |
|
ATTO rho101 |
5′, 3′
|
Rhodamine derivative |
|
Dual-labelled fluorogenic probes for real-time PCR. |
BHQ-2 |
|
ATTO 590 |
5′, internal, 3′
|
Rhodamine derivative |
|
Single molecule detection, high-resolution microscopy, and FISH. |
BHQ-2 |
|
ATTO 594 |
5′, 3′
|
Rhodamine derivative |
|
Single molecule detection, high-resolution microscopy, and FISH. |
BHQ-2 |
|
ATTO 620 |
5′, 3′
|
Rhodamine derivative |
|
Dual-labelled fluorogenic probes for real-time PCR. |
BHQ-2 |
|
ATTO 633 |
5′, internal, 3′
|
Carborhodamine dyes Cationic |
|
Single molecule detection, high-resolution microscopy, and FISH. |
BHQ-3 |
|
ATTO 647N |
5′, internal, 3′
|
Carborhodamine dyes Cationic |
|
Single molecule detection, high-resolution microscopy and FISH. |
BHQ-2 |
|
ATTO 655 |
5′, 3′
|
Oxazine derivative Zwitterionic dye |
|
Single molecule detection, high-resolution microscopy and FISH. |
BHQ-3 |
|
ATTO 680 |
5′, internal, 3′
|
Oxazine derivative Zwitterionic dye |
|
Dual-labelled fluorogenic probes for real-time PCR. |
BHQ-3 dye Fluorescence quenching by electron donors like guanine, tryptophan, etc. |
|
ATTO 700 |
5′, 3′
|
Oxazine derivative Zwitterionic dye |
|
Single molecule detection, high-resolution microscopy, and FISH. | BHQ-3 dye Fluorescence quenching by electron donors like guanine, tryptophan, etc. |
|
ATTO 725 |
5′
|
|
Can be used in a wide array of applications.
|
||
|
ATTO 740 |
5′, 3′
|
|
Can be used in a wide array of applications. |
Know your oligo mod series
- 5-Nitroindole – a universal base oligo modification
- BHQ® (Black Hole Quencher®) non-fluorescent quenchers
- Methylene Blue
- Thio C6 linker
- 3' Spacer C3
- Phosphorothioate bonds
References
-
Park HY, Buxbaum AR, Singer RH. Single mRNA tracking in live cells. Methods Enzymol. 2010. 472:387-406. doi:10.1016/S0076-6879(10)72003-6
-
Sauer M, Zander C, Müller R, Ullrich B, Drexhage K, Kaul S, Wolfrum J. Detection and identification of individual antigen molecules in human serum with pulsed semiconductor lasers. Applied Physics B: Lasers and Optics. 1997. 65(3):427-431. doi:10.1007/s003400050292
-
Wall KP, Dillon R, Knowles MK. Fluorescence quantum yield measurements of fluorescent proteins: a laboratory experiment for a biochemistry or molecular biophysics laboratory course. Biochem Mol Biol Educ. 2015. 43(1):52-59. doi:10.1002/bmb.20837
-
Santos EM, Sheng W, Esmatpour Salmani R, et al. Design of Large Stokes Shift Fluorescent Proteins Based on Excited State Proton Transfer of an Engineered Photobase. J Am Chem Soc. 2021.143(37):15091-15102. doi:10.1021/jacs.1c05039
-
Zheng Q, Lavis LD. Development of photostable fluorophores for molecular imaging. Curr Opin Chem Biol. 2017. 39:32-38. doi:10.1016/j.cbpa.2017.04.017


