Originally published : Tue, January 31, 2023 @ 1:38 PM
Updated : Tue, May 9, 2023 @ 10:51 AM
In the next edition of the “Know your oligo mod” series, we look at a universal base analogue for oligonucleotide synthesis – 5-nitroindole.
A universal base is a nucleotide that can pair equally with all four naturally occurring DNA/RNA bases.1 Ideally, the addition of universal bases to an oligonucleotide does not alter the stabilisation of the adjacent base pairs and must retain the structure and function of the oligonucleotide duplex.
Universal bases were primarily developed as degenerate PCR primers, and probes that target partially known sequences. Several compounds such as hypoxanthine, nitroazoles, isocarbostyril analogues, azole carboxamides and aromatic triazole analogues have been employed as universal bases and used for degenerate PCR, microarray probes, ligation and triplexes.2
Discovery of 5-nitroindole as a universal base
The first described universal base was hypoxanthine, which is also known as inosine when in nucleotide form. However, the hypoxanthine structure closely resembles guanine and forms the most stable base pair with cytosine. This inherent bias led to further research into designing new base analogues with a more “universal” nature.
In 1994, two new universal aromatic, hydrophobic base analogues were described, namely, the 2’-deoxyribosyl-derivatives of 3-nitropyrrole and 5-nitroindole. Both these compounds have no hydrogen-bonding property but instead have a stacking ability within the double helix DNA. Therefore, they exhibit an equal base-pairing capacity with all the four bases.2,3 Comparative studies further revealed that 5-nitroindole derivative is superior giving higher duplex stability even with multiple substitutions.4,5
| A) |
B) |
|
|
|
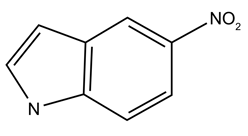
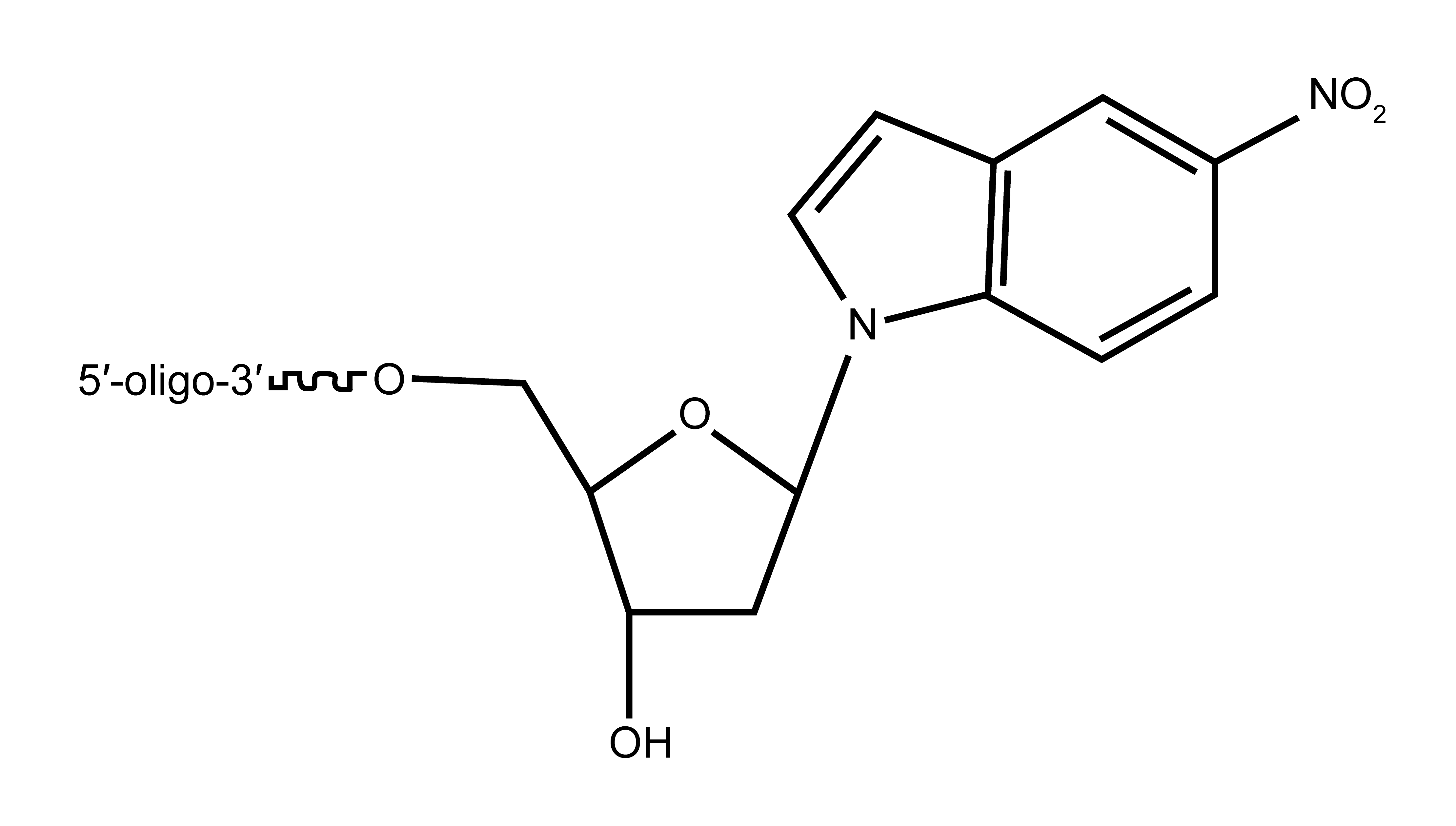
Figure 1: (A) Structure of 5-nitroindole comprising aromatic ring structure and (B) the deoxyribosyl-derivative of 5-nitroindole.
Properties of 5-nitroindole base analogue
5-nitroindole is considered to be one of the best universal bases for increased stability of the duplex through base-stacking interactions. These interactions have been well studied and validated, and the enhanced stacking ability is attributed to their larger aromatic surface area3 and increased hydrophobicity.6 Nuclear magnetic resonance spectroscopy has shown that 5-nitroindole universal bases are fully stacked within DNA duplexes, adopting an anti conformation that provides good overlap with adjacent bases.2
Also, melting temperature (Tm) experiments demonstrate that the incorporation of 5-nitroindole is much less destabilising than other compounds. Only a slight decrease in Tm was observed when incorporated towards the end (2 oC) or in the middle (5 oC) of a 17-mer duplex (with an unmodified duplex Tm of 72 oC). The analogue pairs equally well opposite all four natural bases, with a ΔTm range of 3 oC.6 In addition, Tm studies of DNA hairpins with 5-nitroindole bases in the loop region were also shown to be more stable than the control hairpin with a T4 loop.2 These properties give 5-nitroindole an important advantage for applications based on hybridisation.
Applications of 5-nitroindole modified oligos
The improved hybridisation property of 5-nitroindole modified oligonucleotides have found varied applications in biotechnology. Some of the common applications and criteria to optimise efficiency are discussed here.
Probes
5-nitroindole is ideal for designing nested probes to target regions of the rRNA. They have been successfully employed to target rRNA for different microbial species with equal specificity. They are also useful in studying protein-DNA interactions, for example, nucleotide excision repair mechanisms.6 Interestingly, incorporation of 5-nitroindole to one or both ends of the hexamers in oligonucleotide chips demonstrated improved performance.
Primers
Universal bases are used within primers for PCR and sequencing. Although 5-nitroindole may stabilise the primer/template/polymerase structure, it has been found that primers containing 5-nitroindole within the first 7-8 bases from the 3′-end reduce PCR efficiency.6 Moreover, priming for DNA synthesis can be poor when more than two substitutions are made at codon third positions, or >4 substitutions occur in the middle or 5′-end. However, the inclusion of up to four contiguous 5-nitroindole bases in the middle or 5′-end can be made, and these will prime DNA synthesis in PCR and sequencing.6
Universal nucleoside triphosphates
While 5-nitroindole analogues are not good substrates for Klenow and Taq DNA polymerases, they work well with terminal deoxynucleotidyl transferase and produce better tailing than dATP.6
Furthermore, several experiments have demonstrated the use of 5-nitroindole modified nucleotides in biomedical research applications. For example, incorporation of 5-nitroindole nucleotides into an siRNA passenger strand can reduce the off-target effects of RNA-induced silencing complex (RISC).7 Similarly, a single modification of 5-nitroindole in the Thrombin-binding aptamer HD1 enhanced the anticoagulant properties and binding affinity of the aptamer.8,9
In summary, the use of universal bases in biotechnology has been growing. While research continues to develop a more effective universal base, 5-nitroindole is currently the most widely used universal base analogue.
Research grade to commercial use
LGC Biosearch Technologies™ offers custom manufacturing of oligonucleotides with an expansive portfolio of modifications. With ISO 9001 or ISO 13485 synthesis, scalability and multi-site manufacturing, you can be sure we’ll meet your exact needs.
Explore our oligos page to learn more about our custom oligo synthesis and full range of products, or configure your oligo here. For technical assistance regarding oligo modifications, contact techsupport@lgcgroup.com.
How to order
If you are based in the Americas or APAC
Primers and custom oligos : Use the Order now tab to configure and order your oligo(s) using our online tool. Select from the internal modification dropdown list or insert “[5-Nitroindole]” into your sequence for each addition of 5-nitroindole.If you are ordering many oligos or require orders of >1 μmol synthesis scale, consider using the Build Your Oligo excel order form with online upload and email submission options.
Probes : Select the correct excel order form for your probe type and insert [5-Nitroindole] into your probe sequence for each addition of 5-nitroindole. The option to order primers (with or without 5-nitroindole) is not mandatory. Follow the instructions in the excel order form to complete your order and send to info@biosearchtech.com .
If you are based in EMEA
Primers, custom oligos, and probes: Select the correct excel order form for your oligo type and insert [5-Nitroindole] into your sequence for each addition of 5-nitroindole. An option is available to order primers (with or without 5-nitroindole) with the purchase of probes, but this is not mandatory. Follow the instructions in the excel order form to complete your order and send to lys.eu@lgcgroup.com.
More from the "Know your oligo mod" series
BHQ™ (Black Hole Quencher) non-fluorescent quenchers
References
- Loakes D, Hill F, Brown DM, Salisbury SA. Stability and structure of DNA oligonucleotides containing non-specific base analogues. J Mol Biol. 1997;270(3):426-435. doi:1006/jmbi.1997.1129. Published 1997. Accessed December 9, 2022.
- Liang F, Liu YZ, Zhang P. Universal base analogues and their applications in DNA sequencing technology. RSC Advances, 3(35), 14910–. doi:1039/C3RA41492B. Published 2013. Accessed December 9, 2022.
- Herdewijn P. Modified Nucleosides: Universal Base Analogues and their Applications to Biotechnology. 1002/9783527623112(), 277–303. doi:10.1002/9783527623112.ch11. Published 2008. Accessed December 9, 2022.
- Loakes D, Brown DM. 5-Nitroindole as an universal base analogue. Nucleic Acids Res. 1994;22(20):4039-4043. doi:1093/nar/22.20.4039. Published 2008. Accessed December 9, 2022.
- Loakes D, Brown DM, Linde S, Hill F. 3-Nitropyrrole and 5-nitroindole as universal bases in primers for DNA sequencing and PCR. Nucleic Acids Res. 1995;23(13):2361-2366. doi:1093/nar/23.13.2361. Published 1995. Accessed December 9, 2022.
- Loakes D. Survey and summary: The applications of universal DNA base analogues. Nucleic Acids Res. 2001;29(12):2437-2447. doi:1093/nar/29.12.2437. Published 2001. Accessed December 9, 2022.
- Zhang J, Zheng J, Lu C, Du Q, Liang Z, Xi Z. Modification of the siRNA passenger strand by 5-nitroindole dramatically reduces its off-target effects. Chembiochem. 2012;13(13):1940-1945. doi:1002/cbic.201200349. Published 2012. Accessed December 9, 2022.
- Kolganova NA, Tsvetkov VB, Smirnov IP, Timofeev EN. Probing the Nitroindole-Modified Central Loop of Thrombin Aptamer HD1 as a Recognition Site. Nucleic Acid Ther. 2019;29(4):208-217. doi:1089/nat.2018.0757. Published 2019. Accessed December 9, 2022.
- Tsvetkov V, Varizhuk A, Pozmogova G. et al. A Universal Base in a Specific Role: Tuning up a Thrombin Aptamer with 5-Nitroindole. Sci Rep 5, 16337 (2015). doi:10.1038/srep16337. Published 2015. Accessed December 9, 2022.