Originally published : Thu, July 23, 2015 @ 5:33 PM
Updated : Friday October 17, 2025 @ 4:03 PM
Black Hole Quenchers™ (BHQ™) are among the most popular non-fluorescent quenchers for hydrolysis probes thanks to their low background fluorescence. These quenchers come in a variety of forms, so this blog post will cover the basics of how they work and how to choose the right quencher for your applications.
Before exploring the various BHQ dye modifications, we should briefly explain what quenchers are and how they are used in oligonucleotides.
FRET quenching
Quenchers are a class of molecules that can suppress fluorescence emission through a mechanism known as fluorescence resonance energy transfer, or FRET.
An excited reporter dye can transfer its energy to a nearby quencher without any light emission or absorption. This process requires the emission spectrum of the reporter dye to have sufficient overlap with the absorbance spectrum of the quencher dye.
This transfer returns the reporter dye to its ground state and the quencher dissipates the energy. Dark quenchers, like BHQ, release this energy as heat as they have no native fluorescence themselves. Other quenchers, such as tetramethylrhodamine (TAMRA), emit their own fluorescence, which can contribute to background signal in an assay.
The reporter and quencher are placed at specific sites in an oligonucleotide so that a change in their distance will produce a change in fluorescence, signalling the event being monitored (often hybridisation or nuclease activity).
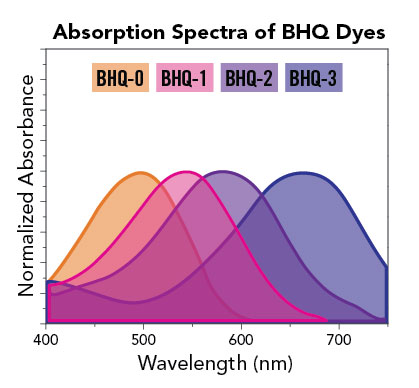
As shown in the absorption spectra below, a variety of BHQ options are available to pair with dyes ranging in wavelength from 400 nm to 750 nm.
Static quenching
BHQ dyes can also efficiently operate via static (contact) quenching mechanisms with some dye pairings.1-3 This occurs when hydrophobic and electrostatic interactions facilitate the association of BHQ dyes with certain reporters to form an intramolecular dimer, resulting in enhanced quenching and an improved signal-to-noise ratio.
Both FRET quenching and static quenching can occur together. The static quenching mechanism means that BHQ dyes may quench a fluorophore whose emission spectrum lies beyond the limits of BHQ absorption.
The efficiency of static quenching depends on the affinity between the reporter and the quencher. The reporter and quencher often are planar, hydrophobic molecules that stack together to avoid contact with the surrounding water.
Selecting the appropriate non-fluorescent quencher
When choosing the correct quencher for a probe, you normally want to match the absorption range the fluorescent reporter’s emission.
However, the impact of static quenching can affect the recommendations for some dye-quencher pairings. For example, our Quasar® 670 and Quasar 705 dyes partner best with the BHQ-2 quencher, even though the BHQ-3 quencher appears to have the optimal spectral overlap. Therefore, certain dyes may have multiple effective quencher options.
If you are unsure about which quencher to choose, our PCR Forge tool can help you make these decisions. It is the only PCR design tool that can handle the full BHQ range to create probes for qPCR, SNP genotyping and gene expression assays.
High-performance BHQ probes
 The BHQplus™ probe is a compact probe for qPCR with a duplex stabilising technology terminated with the BHQ dye.
The BHQplus™ probe is a compact probe for qPCR with a duplex stabilising technology terminated with the BHQ dye.
BHQplus probes use modified C and T nucleotides to stabilise the duplex, allowing you to use shorter oligonucleotides. This provides greater specificity and can help detect more difficult targets, such as AT-rich regions and single nucleotide polymorphisms.
Shorter probes - BHQplus
When a long probe (over 25 bases) is necessary, such as to achieve a suitable melting temperature in an AT-rich target region, the increased distance between the fluorophore and quencher can reduce the quenching efficiency in regular end-labelled probes.
 BHQnova™ qPCR probes incorporate an additional Nova quencher placed internally between bases 9 and 10 from the 5’ end of the oligo. This extra quenching moiety between the 5’ fluorophore and the 3’ BHQ allows for much longer probe sequences, 25 bases and beyond, while maintaining excellent quenching characteristics.
BHQnova™ qPCR probes incorporate an additional Nova quencher placed internally between bases 9 and 10 from the 5’ end of the oligo. This extra quenching moiety between the 5’ fluorophore and the 3’ BHQ allows for much longer probe sequences, 25 bases and beyond, while maintaining excellent quenching characteristics.
Longer probes - BHQnova
The signal release is unaffected by the addition of the Nova quencher, often resulting in enhanced signal-to-noise ratios compared to standard dual-labelled probes.
Although BHQnova probes were designed to improve the performance of long probes, they can also be used for standard length probes. The additional nova quencher helps to further reduce background fluorescence, providing unparalleled signal-to-noise ratio and clear results.
BHQnova probes are compatible with other probe formats in multiplex assays. Barring any sequence interactions, you can combine BHQnova probes with standard BHQ probes or BHQplus probes in the same reaction.
Available BHQ modifications for custom oligonucleotides
LGC Biosearch Technologies™ offers multiple versions of our BHQ dye modification to allow different quencher positions (see table below). All BHQ dyes are available as terminal 5’ and 3’ modifications and many are also available in internal positions, which come in two versions:
- Standard internal BHQ modifications have an abasic linkage through the sugar-phosphate backbone of the oligo. This version of the internal BHQ may affect oligonucleotide geometry upon hybridisation because it sits between the standard base linkages on either side.
- The T(BHQ) version of the internal modification has the quencher attached to a deoxythymidine (dT) nucleoside to allow continuity of the oligonucleotide backbone. This minimises the potential disruption of hybridisation by linking the quencher off the base.
| Dye | Effective quenching range |
5’ Mod. | 3’ Mod. | Internal mod. |
| BHQ-0 | 430-520 nm | ✔ | ✔ | |
| BHQ-1 | 480-580 nm | ✔ | ✔ | ✔ |
| T(BHQ-1) | 480-580 nm | ✔ | ✔ | |
| BHQ-2 | 559-670 nm | ✔ | ✔ | ✔ |
| T(BHQ-2) | 559-670 nm | ✔ | ✔ | |
| BHQ-3 | 620-730 nm | ✔ | ✔ | ✔ |
Designing Black Hole Quencher probes
The wide variety of BHQ modifications and probe types mean that there is a solution to almost any application. For help with designing probes that are customised to your needs, try PCR Forge. This free, web-based software is the only assay design tool to specifically design BHQ, BHQplus and BHQnova probes.PCR Forge delivers probe designs you can trust, so you can spend less time designing and more time focusing on your research.
References
- Marras SAE, Kramer FR and Tyagi S (2002) Efficiencies of fluorescence resonance energy transfer and contact-mediated quenching in oligonucleotide probes. Nucleic Acids Research 30(21):e122 doi: 10.1093/nar/gnf121
- Johansson MK et al. (2002) Intramolecular Dimers: A New Strategy to Fluorescence Quenching in Dual-Labeled Oligonucleotide Probes. J Am Chem Soc 124:6950 doi: 10.1021/ja025678o
- Johansson MK (2006) Choosing Reporter-Quencher Pairs for Efficient Quenching Through Formation of Intramolecular Dimers. Methods Mol Biol 335:17 doi: 10.1385/1-59745-069-3:17


