Originally published : Tue, November 14, 2023 @ 5:59 PM
While RNA-based therapeutics are taking the biotechnology world by storm following the rapid development of the COVID-19 vaccines, the next generation of treatments could already be here.1 Despite being the lesser-known cousin of linear mRNA, circular RNA (circRNA) holds tremendous potential for emerging pharmaceutical applications by increasing the stability of the therapeutics and lowering manufacturing costs.
What is circular RNA?
 circRNA is naturally produced by eukaryotic cells and consists of a single-stranded RNA with covalently linked 5ʹ and 3ʹ ends. There is still a lot to learn about the roles of these molecules in the cell, which have drawn interest as protein/gene sponges, cell activity modulators and disease biomarkers.1
circRNA is naturally produced by eukaryotic cells and consists of a single-stranded RNA with covalently linked 5ʹ and 3ʹ ends. There is still a lot to learn about the roles of these molecules in the cell, which have drawn interest as protein/gene sponges, cell activity modulators and disease biomarkers.1 Advantages for developing therapeutics
The covalently-closed structure of circRNAs offers a significant stability advantage over linear mRNA for therapeutic applications. Without 5ʹ or 3ʹ ends, circular RNAs are more resistant to exonuclease degradation than linear RNA.2 This increased stability may promote pharmacokinetic accumulation in various cell or tissue types. This characteristic could mean that lower doses are required for therapeutic impact, which would reduce manufacturing costs and potentially improving patient safety.1 In addition, without a 5ʹ end, circRNA doesn’t require a capping agent, which accounts for about 40 percent of the spend on mRNA raw materials, making circRNA considerably less expensive to manufacture than linear mRNA.3

Considerations for circRNA therapeutics developers
Engineered circRNA can be synthesised using one or multiple precursor linear RNAs that are circularised by chemical or enzymatic ligation.6 However, several obstacles must be overcome to use circular RNAs successfully in therapeutic applications: finding efficient circularisation methods for long in vitro transcribed (IVT) RNA, ensuring sufficient protein expression, and purifying circRNA adequately.5
Circularisation efficiency optimisation
Translation efficiency optimisation
- May improve circRNA protein yields by several hundred-fold
- Provide increased translation compared to mRNA in vitro
- Deliver more durable translation in vivo
- Are generalisable across multiple transgenes8
 circRNA purification
circRNA purification
Another aspect that may limit therapeutic utility is purity. Impure circRNA may induce strong immune responses, adding safety risks for patients.9 RNase R is a 3′ to 5′ exoribonuclease with helicase activity that digests most linear RNAs, while leaving circRNA untouched. This makes it invaluable for removing precursor linear RNA that remains after the circularisation process, helping to purify the therapeutic circRNA.8
Exciting applications of circRNA in therapeutics
|
|
|
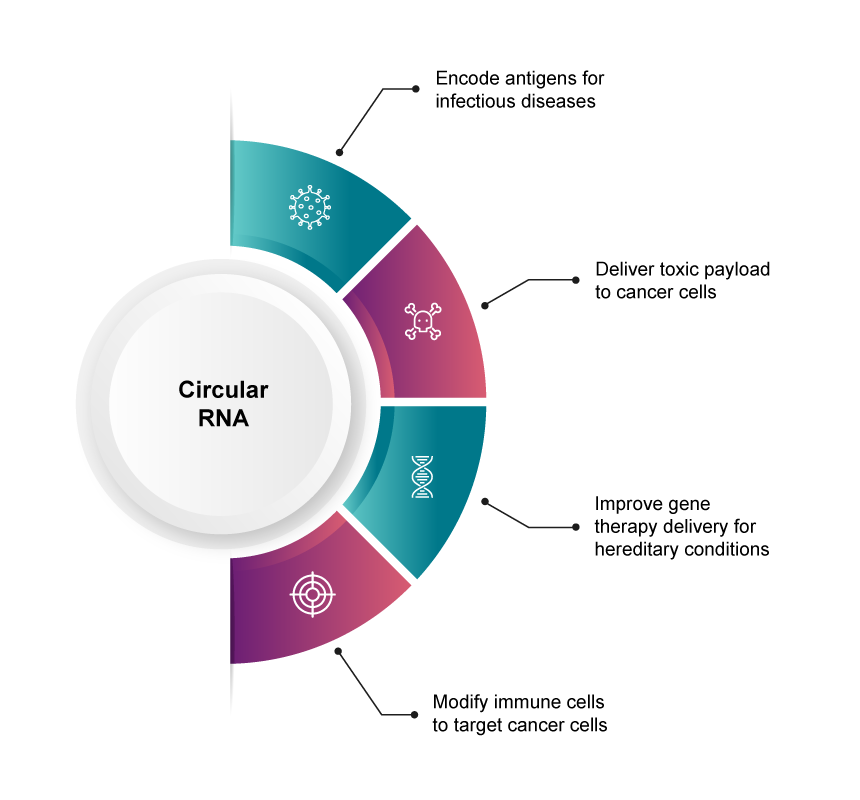
The molecular characteristics of circular RNA make it a useful candidate for various therapeutic applications, including protein and peptide replacement, vaccines, and biosensors.1 Many academic groups and biotechnology companies are currently investigating circRNA therapeutics for a range of indications, including:
- Orna™, a therapeutics company specialising in circRNA products, uses the technology to create modified immune cells within a patient (in situ CAR therapy) and deliver improved gene therapy for Duchenne Muscular Dystrophy.
- Circio, a therapeutics company specialising in circRNA medications for cancer and rare diseases, has created an immunotherapy program that uses a patient’s immune system to fight cancer cells.
- A joint effort between Ginkgo Bioworks and Esperovax aims to develop circRNAs that specifically target colorectal cancer cells to induce the expression of a toxic payload that causes cell death. The novel treatment reduces toxicity and the resulting side effects from the death of non-cancerous cells.
- Researchers have developed a circRNA vaccine that encodes the SARS-CoV-2 receptor-binding domain. The team successfully induced potent and sustained neutralising antibodies via the LNP-encapsulated vaccine, which was reported to be highly heat-stable and could help to reduce cold-chain limitations.4
- Liu X, Zhang Y, Zhou S et al. Circular RNA: An emerging frontier in RNA therapeutic targets, RNA therapeutics, and mRNA vaccines. J Control Release. 2022. 348:84-94. doi: 10.1016/j.jconrel.2022.05.043
- Abe H, Abe N, Uda M et al. Synthetic nanocircular RNA for controlling of gene expression. Nucleic Acids Symp. Ser. Oxf. 2009. 65–66. doi: 10.1093/nass/nrp033
- Grand View Research. mRNA Synthesis Raw Materials Market Size, Share & Trends Analysis Report By Type (Capping Agents, Nucleotides, Plasmid DNA), By Application, By End-user, By Region, And Segment Forecasts, 2023 - 2030. https://www.grandviewresearch.com/industry-analysis/mrna-synthesis-raw-materials-market-report
- Qu L, Yi Z, Shen Y et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell. 2022. 185(10):1728-1744.e16. doi: 10.1016/j.cell.2022.03.044
- Wesselhoeft RA, Kowalski PS and Anderson DG. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 2018. 9:2629. doi: 10.1038/s41467-018-05096-6
- Dolgin E. Why rings of RNA could be the next blockbuster drug. Nature. 2023. 622, 22-24 doi: 10.1038/d41586-023-03058-7
- Petkovic S and Muller S. Synthesis and engineering of circular RNAs. Methods Mol. Biol. 2018. 1724:167–180. doi: 10.1007/978-1-4939-7562-4_14
- Chen R, Wang SK, Belk JA et al. Engineering circular RNA for enhanced protein production. Nat Biotechnol. 2023. 41: 262–272. doi: 10.1038/s41587-022-01393-0
- Wesselhoeft RA, Kowalski PS, Parker-Hale FC et al. RNA circularization diminishes immunogenicity and can extend translation duration in vivo. Mol. Cell. 2019. 74(3):508–520 e4. doi: 10.1016/j.molcel.2019.02.015
- Xiao MS and Wilusz JE. An improved method for circular RNA purification using RNase R that efficiently removes linear RNAs containing G-quadruplexes or structured 3' ends. Nucleic Acids Res. 2019. 47(16):8755-8769. doi: 10.1093/nar/gkz576
- Lee KH, Kim S and Lee S-W. Pros and cons of in vitro methods for circular RNA preparation. Intl J of Mol Sci. 2022. 23(21):13247. doi: 10.3390/ijms232113247


