Originally published : Wed, April 10, 2013 @ 10:30 PM
Updated : Wed, October 1, 2014 @ 4:53 PM
Stellaris probe sets targeting glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA were among the first designed at Biosearch Technologies. Due to the long history of its use as a control in northern blots and real time qPCR, GAPDH was a natural choice for an initial housekeeping gene. The use of probes targeting GAPDH in Stellaris assays, however, is somewhat more nuanced. GAPDH is highly expressed in most all cell types – in our tests of the probe set in A549 cells, we counted upwards of 2000 copies per cell. With such high expression, the GAPDH probe set is ideal for testing filter set and microscope compatibility, as well as assay preparation. Positive signal is immediately obvious due to the high number of transcripts per cell.
On the other hand, the GAPDH probe set is not ideal for testing spot counting software. Some of the more sophisticated algorithms can make good guesses, but the large number of overlapping spots from spatially close transcripts poses some challenges in image analysis. For a positive control that is more amenable to being counted by automated software, it is preferable to choose a gene with lower expression, such as the mRNA for RNA polymerase II (POLR2A).
The GAPDH gene product is an enzyme essential to carbohydrate metabolism. There are many pseudogenes related to GAPDH throughout the human genome, and one the testis specifically expressed GAPDHS paralog. The GAPDH probe set in our catalog is specific to the somatically expressed GAPDH, and does not detect the GAPDHS paralog. The expected expression pattern is a high concentration of transcripts throughout the cytoplasm, with some cells also exhibiting bright spots at the sites of active transcription in the nucleus.
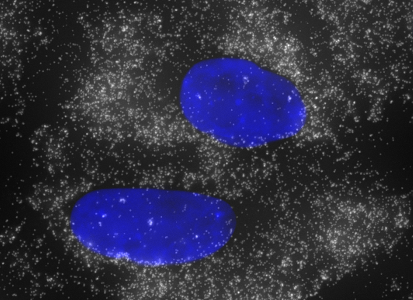
GAPDH in A549 Cells
- GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. (PubMed id 15769908) Barber R.D....Clark B.J. (2005)
- Subcellular localization of human glyceraldehyde-3-phosphate dehydrogenase is independent of its glycolytic function. (PubMed id 12829261) Mazzola J.L. and Sirover M.A. (2003)

