Originally published : Wed, January 6, 2010 @ 5:31 PM
Updated : Thu, September 22, 2022 @ 5:16 PM
1. Instrumentation and Dye Selection
This one is pretty easy. For a successful multiplex, your instrument needs to be able to excite each of your reporter dyes. Although there are dyes on the market that excite in the far red, you'll be safe using an instrument which excites over the visible spectrum (350-750nm). Biosearch Technologies has developed two essential tools to assist you with dye selection:
- An interactive spectral overlay tool that allows you to compare spectra and pair fluorophores with BHQ™ dyes, with recommendations tailored to your instrument.
- Multiplexing recommendations
2. Probe and Primer Design
For a successful qPCR multiplex experiment, primers and probes can be designed with software programs that specialise in creating assays that avoid secondary structures and primer-primer interactions. While there isn’t always a choice in the target sequence, maximising the length of the target sequences, when possible, increases the likelihood a compatible assay will be found within the targets. If creating an assay that covers four or more channels, assays for abundant targets (like control assays) can be designed with probes with red shifted dyes (see interactive spectral overlay tool to review which dyes are best suited for your qPCR instrument), reserving brighter dyes (eg. FAM and CIV-550) for the detection of less abundant test target.
3. Dealing with Crosstalk
Dyes should be chosen with distinct emission and excitation spectra in order to minimise crosstalk. Crosstalk is fluorescent signal bleed-through between channels. Although unavoidable, crosstalk can be controlled by calibrating your instrument before running the experiment. LGC Biosearch Technologies™ offers calibration standards that enable the instrument to store the fluorescent profile of each dye, and SuperRoxTM dye for use as a passive reference to normalise for signal variation.
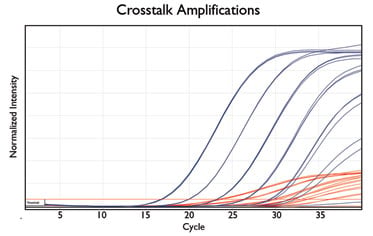
Figure showing signal bleeds from CAL Fluor® Red 610 dye(red traces) into the channel detection Quasar® 670 dye (blue traces). Crosstalk is subsequently removed using software settings.
That's pretty much all you need to know to help you get a quick jump start in developing a multiplexed qPCR assay.
Need help?
If you would like help creating a multiplex assay, please contact our Assay Design and Development team at ADD@LGCgroup.com to discuss your assay needs. Or check out our FAQs page.


