Originally published : Thu, September 25, 2014 @ 3:48 PM
Updated : Mon, September 19, 2022 @ 2:13 PM
The largest recorded Ebola Virus Disease outbreak in history continues to devastate the West African region. This epidemic has presented a public health emergency to national and international disease control centers with aid workers tirelessly working to assist affected communities. Ebola is a single-stranded RNA virus that has five known strains, four of which are pathogenic to humans. The current outbreak is a result of the spread of the Zaire ebolavirus (EBOV) strain which previously averaged a 78% mortality rate 1. In a recent update from the World Health Organization, upwards of 2,600 deaths have been reported as result of the viral infection over the last several months 2.
In order to prevent the further proliferation of the epidemic, clinicians are utilizing medical diagnostics to assist in the rapid identification of Ebola strains; expediting the quarantine and treatment of infected patients. The current detection methods at the disposal of the scientist include viral culture, electron microscopy (TEM), and antibody-based detection such as immunohistochemistry and ELISA 3. However, the Centers for Disease Control and Prevention are indicating the use of probe-based RT-qPCR assays for acute infections 4. RT-qPCR detection was used in previous outbreaks as well as the current crisis as an sensitive and reliable means to identify the virus in potentially infected individuals 5.
In response to the current outbreak, the US Food and Drug Administration authorized the emergency use of the U.S. Department of Defense (DoD) EZ1 Real-time RT-PCR assay as one of the approved diagnostic methods for confirming presence of the Ebola virus 5. The assay is provided only through the US Department of Defense to select laboratories designated to handle such potentially hazardous samples. Patients suspected of infection or exposure are able to obtain results from their tested blood sample within a matter of hours due to the nature of the assay. The qPCR detection method, in part, relies upon the use of a dual-labeled FRET probe containing a reporter dye and quencher to quantitatively confirm the presence of the viral cDNA. The speed and sensitivity of these probe-based qPCR assays have made them amenable to rapid diagnostics in the face of past outbreaks, such as the H1N1 pandemic in 2009. As for the current situation with EBOV in West Africa, it is hoped that the efforts of the international coalition of aid workers can stem the tide and contain the spread of deadly disease.
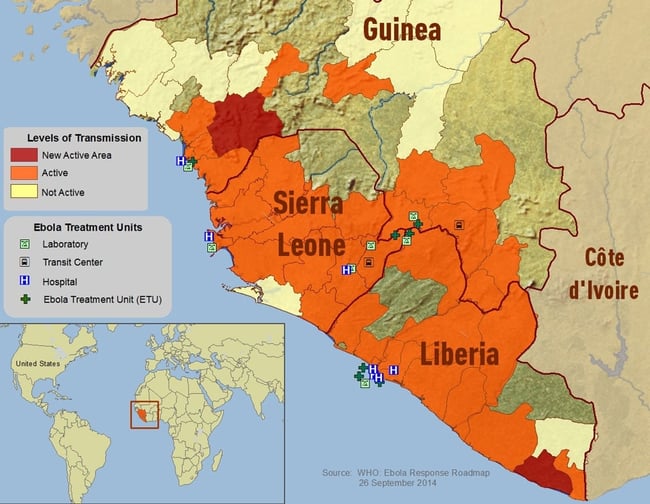
Map of affected region 9/14/14: Source CDC/VSBP; E.Ervin
References:
1Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Gire SK, et al. Science. 2014 Aug
2WHO: Ebola Response Roadmap Update. Accessed: 9/18/2014
3Development and evaluation of a fluorogenic 5’ nuclease assay to detect and differentiate between Ebola virus subtypes Zaire and Sudan. Gibb TR, Norwood DA Jr, Woollen N, and Henchal EA. J Clin Microbiol. 2001 Nov; 39(11):4125-4130
4CDC: Interim Guidance for Specimen Collection, Transport, Testing, and Submission for Persons Under Investigation for Ebola Virus Disease in the United States. Accessed: 9/10/2014
5Emergence of Zaire Ebola virus disease in Guinea—Preliminary report. Baize S, et al. N Engl J Med. 2014 Apr
6FDA website for Emergency Use Authorizations. Accessed: 9/10/2014

