Originally published : Thu, September 14, 2023 @ 4:10 PM
As the world scrambled to rapidly expand PCR testing capacity in 2020, it became increasingly clear that there was a need for cheaper, simpler, quicker diagnostic methods that could be deployed at the point of care.1 While its results might be considered the gold standard, PCR requires highly trained staff as well as specialised equipment and facilities. These requirements have limited the use of PCR-based diagnostics in settings where cost and convenience are paramount.
While its results might be considered the gold standard, PCR requires highly trained staff as well as specialised equipment and facilities. These requirements have limited the use of PCR-based diagnostics in settings where cost and convenience are paramount.
LAMP (loop-mediated isothermal amplification of DNA) has become one of the front-runners to meet the need where PCR isn’t feasible. PCR testing requires a cumbersome and expensive thermal cycler to deliver high performance. By contrast, LAMP is an isothermal process requiring only a small, light heating element to maintain temperature, usually 60-65° C.2
Its simplicity makes LAMP ideal for rapid testing in the field or in areas with low resources. It is already helping to detect infectious diseases around the world, including Ebola and Zika.3,4 Several research teams have also developed LAMP tests for COVID-19 infections that demonstrated comparable accuracy to PCR diagnostics.5-7
How LAMP works
- The forward inner primer hybridises to the target DNA and initiates complementary strand synthesis.
- The elongated forward inner primer is displaced by the forward outer primer that hybridises to an upstream target region.
- The inner primers include a reverse complementary sequence designed so that they form a self-hybridising loop.
- The elongated forward inner primer acts as a template for the backwards inner primer and the process is repeated from the opposite end of the target sequence.
- The resulting product resembles a dumbbell as it forms loops at both ends. This creates multiple sites for initiation of synthesis, rapidly amplifying the DNA.
- As synthesis continues, the products form long concatemers that exponentially increase amplification.
- Two additional loop primers can be used to accelerate this process further.
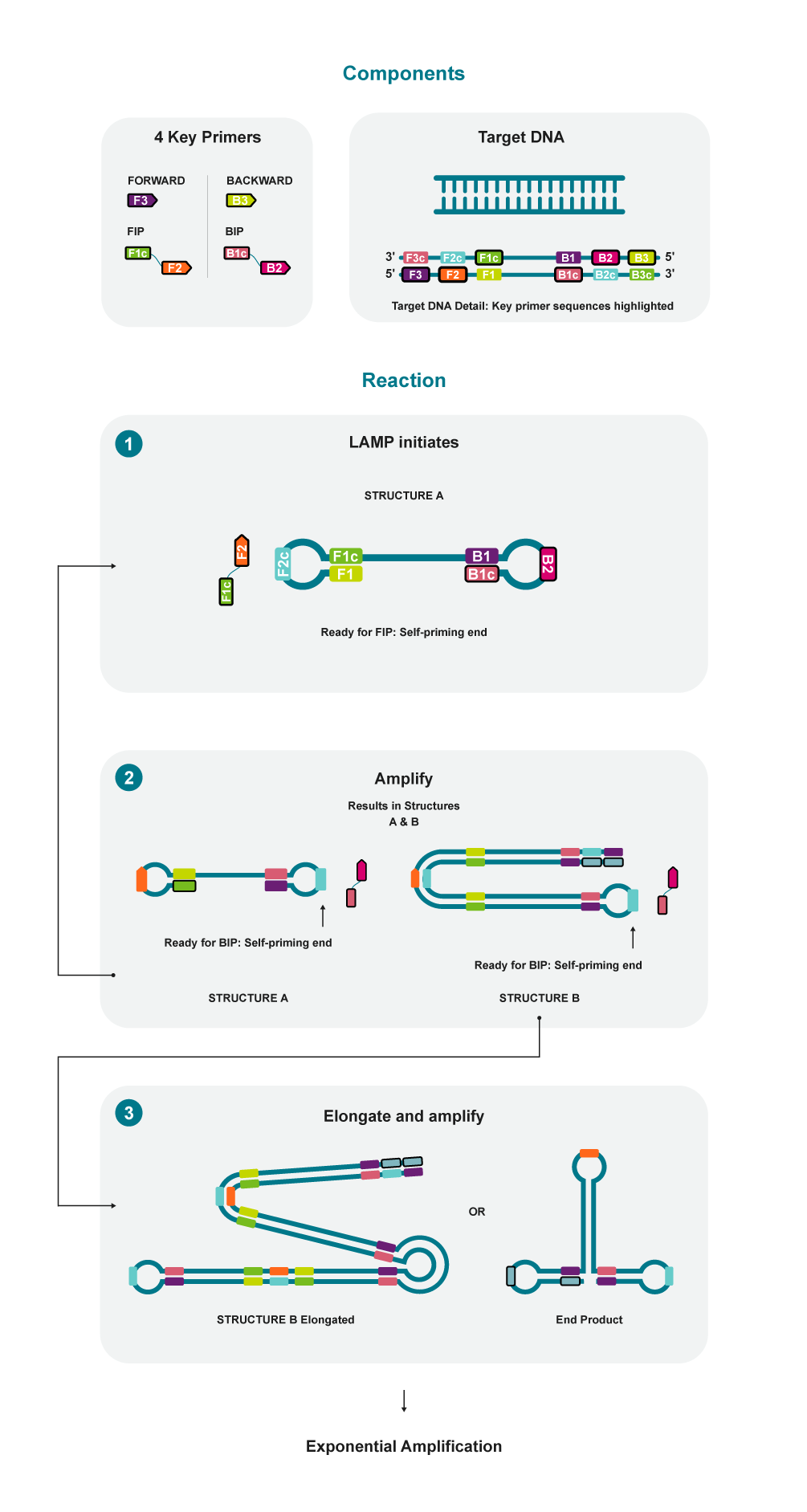 |
| Figure 1. Simplified overview of the loop-mediated isothermal amplification (LAMP) process. For simplicity, 4/6 potential LAMP primers are illustrated. Including two additional primers, F-Loop (Forward Loop Primer) and B-Loop (Backward Loop Primer), often significantly enhances amplification. |
Advantages of LAMP for point-of-need testing
- Simpler preparation – The resilient Bst enzyme used in LAMP can function even in the presence of PCR inhibitors often found in samples like saliva.8 This means that it’s possible to carry out the test without extraction and purification steps.
- No cold supply chain – Reagents for LAMP can be lyophilised, keeping them stable at room temperature. This means that users can avoid the costs and logistical challenges of cold chains, making it even easier to use in areas with limited resources.
- Quick reaction times – The design of LAMP leads to the production of significantly more DNA than PCR-based amplification. This means that it can be performed in less than 30 minutes, offering faster results at the point of need.
- Visible results possible – The result can even be detected by eye, further reducing turnaround time. For higher sensitivity, intercalating dyes can be added to easily monitor the progression of the reaction.
LAMP as a portable respiratory test
Health technology company ProtonDx has taken advantage of the benefits of LAMP in producing its portable testing platform, Dragonfly. This lightweight system can deliver results in less than 30 minutes with a visual colour change.
This means that it “can be as sensitive as traditional PCR but with the advantage of speed,” says Dr Jesus Rodriguez Manzano, co‑founder and CSO of ProtonDx.
 The Dragonfly Respiratory Panel can detect SARS-CoV-2, influenza A, influenza B, respiratory syncytial virus and human rhinovirus. This provides rapid results in a range of settings, including care homes or GP clinics. The system was even used in the 2022 Commonwealth Games to improve treatment decisions and reduce unnecessary antibiotic use.
The Dragonfly Respiratory Panel can detect SARS-CoV-2, influenza A, influenza B, respiratory syncytial virus and human rhinovirus. This provides rapid results in a range of settings, including care homes or GP clinics. The system was even used in the 2022 Commonwealth Games to improve treatment decisions and reduce unnecessary antibiotic use.
As well as testing for several diseases at once, the panel includes extraction and reaction controls to assure proper sample preparation, reagent performance, and environmental and reagent quality.
“Because we are delivering a solution which is comparable to the gold standard, we need to have access to the best reagents. Of course, we have developed a series of in-house solutions to support our technology so far, but it would have taken significantly longer to reach the point where we are at now if we did this for every component. So, for us, it was beneficial to partner with external suppliers, for example reagent suppliers such as LGC Biosearch Technologies, during our proof-of-concept stages,” says Dr Rodriguez Manzano.
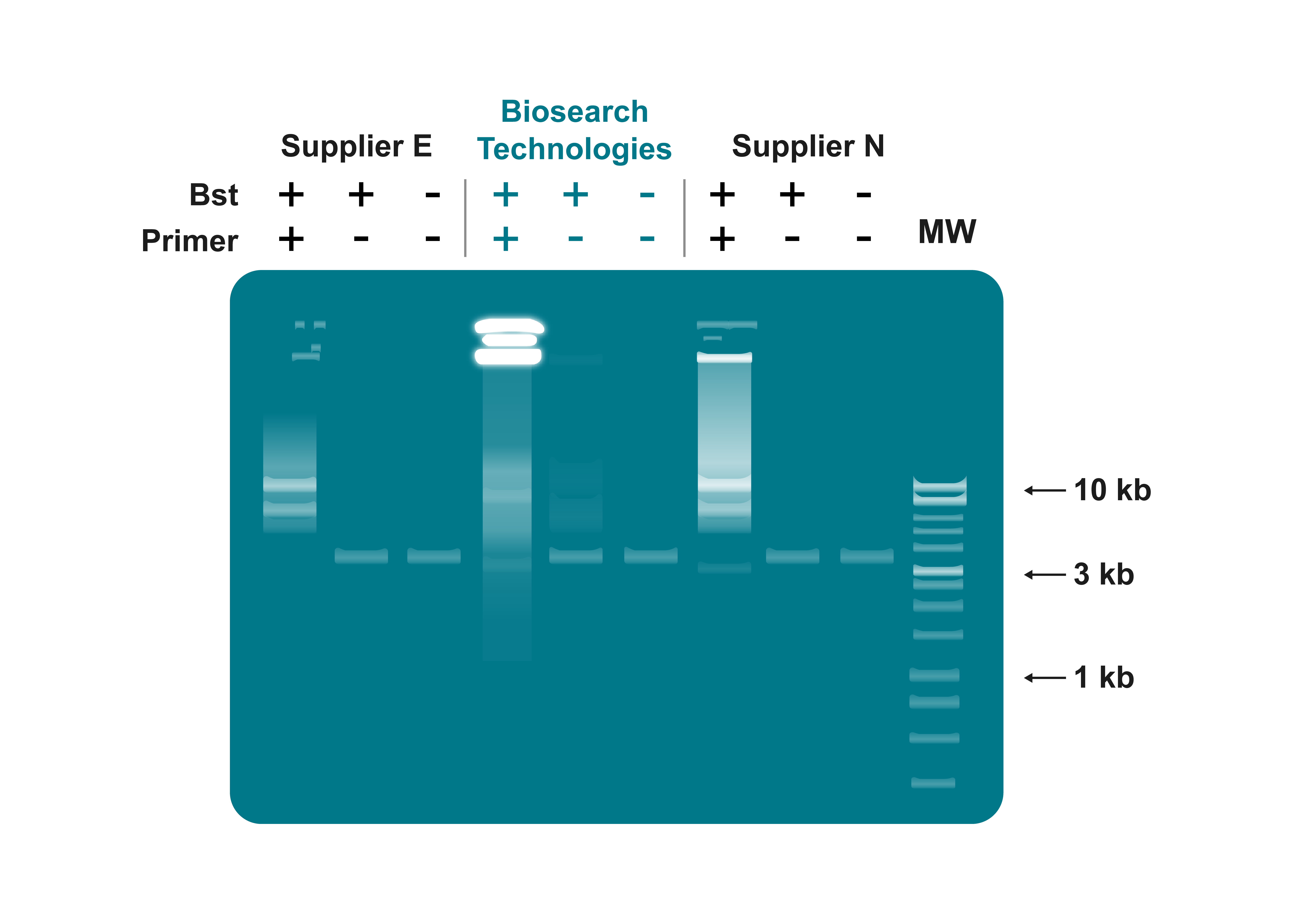 |
| Figure 2. Bst DNA Polymerase, Exonuclease Minus, possesses greater strand-displacing polymerase activity. M13 single-stranded DNA was incubated with or without 8 units of Bst DNA Polymerase(+/- Bst) in reaction buffer supplied by the manufacturer, with or without replication primer (+/- primer) for 30 minutes at 65 °C. MW, 1 kb ladder. |
The potential for LAMP in the future
1. Alhamid G et al. (2023) Development of loop-mediated isothermal amplification (LAMP) assays using five primers reduces the false-positive rate in COVID-19 diagnosis. Scientific Reports. 2023; 13, 5066 https://doi.org/10.1038/s41598-023-31760-z
2. Notomi T, Okayama H, Masubuchi H et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000; 28(12):e63 https://doi.org/10.1093/nar/28.12.e63
3. Bonney LC, Watson RJ, Slack GS, et al. A flexible format LAMP assay for rapid detection of Ebola virus. Plos Neglect Trop D. 2020; 14(7):e0008496 https://doi.org/10.1371/journal.pntd.0008496
4. Sabalza M, Yasmin R, Barber CA et al. Detection of Zika virus using reverse-transcription LAMP coupled with reverse dot blot analysis in saliva. PLOS One. 2018; 13(2):e0192398 https://doi.org/10.1371/journal.pone.0192398
5. Aoki MN, de Oliveira Coelho B, Góes LGB et al. Colorimetric RT-LAMP SARS-CoV-2 diagnostic sensitivity relies on color interpretation and viral load. Scientific Reports. 2021; 11, 9026 https://doi.org/10.1038/s41598-021-88506-y
6. Toppings NB et al. A rapid near-patient detection system for SARS-CoV-2 using saliva. Scientific Reports. 2021; 11, 13378 https://doi.org/10.1038/s41598-021-92677-z
7. Kidd SP, Burns D, Armson B et al. Reverse-Transcription Loop-Mediated Isothermal Amplification Has High Accuracy for Detecting Severe Acute Respiratory Syndrome Coronavirus 2 in Saliva and Nasopharyngeal/Oropharyngeal Swabs from Asymptomatic and Symptomatic Individuals. Journal of Molecular Diagnostics. 2022; 24(4), 320-336 https://doi.org/10.1016/j.jmoldx.2021.12.007
8. Wei S et al. Direct diagnostic testing of SARS-CoV-2 without the need for prior RNA extraction. Scientific Reports. 2021; 11. 2402 https://doi.org/10.1038/s41598-021-81487-y
9. Mannier C and Yoon JY. Progression of LAMP as a Result of the COVID-19 Pandemic: Is PCR Finally Rivaled? Biosensors. 2022; 12(7), 492 https://doi.org/10.3390/bios12070492

