Time to read: 7 minute read
Originally published : Tue, October 3, 2023 @ 10:50 AM
Considering bringing oligo synthesis in-house? Here are some commonly asked questions that we receive, grouped into four sections. You can jump to a section of most interest using this small table of contents here.
- Bringing oligo synthesis in-house
- Selecting the right instrument
- Selecting the right reagents
- Post-synthesis considerations
Listen to more oligo synthesis advice from nucleic acid synthesis expert Lina Borozdina in this webinar.
Q: How can we start oligo synthesis in a basic lab?
Starting oligo synthesis in a basic lab requires meticulous planning, including instrument selection, space and safety considerations, waste management, regulatory compliance, and securing reliable raw material suppliers. Here are the key considerations for in-house oligo synthesis:
- Select the appropriate instrument to meet your projected needs.
- Ensure you have enough space for the instrument and the required ventilation system.
- Ensure you have proper organic solvent storage and disposal systems.
- Verify that your proposed setup will comply with local fire codes.
- Obtain permits for controlled substances, if required.
- Ensure you have the required accessories.
- Verify that you have other necessary equipment.
- List the types of oligos you will be synthesising and identify supplier partners that can reliably deliver the raw materials at the scales you need.
For further information about these considerations, please refer to our e-book.
Q: What are the benefits of synthesising your own oligos?
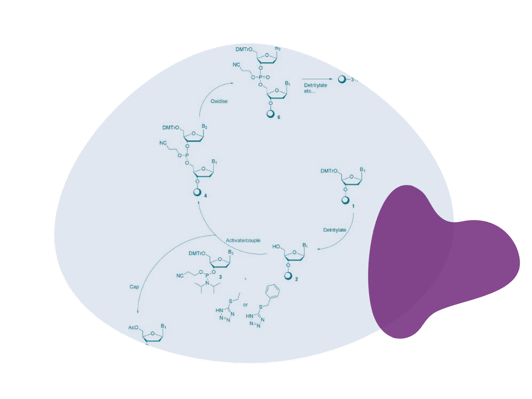 Synthesising your own oligos provides a secure supply chain, faster turnaround times and cost control. Additionally, it allows for customisable synthesis and enhanced quality control, ensuring the accuracy and purity of your oligonucleotides. This approach also offers greater flexibility, enabling researchers to efficiently meet their specific needs while ensuring data integrity and cost-effectiveness.
Synthesising your own oligos provides a secure supply chain, faster turnaround times and cost control. Additionally, it allows for customisable synthesis and enhanced quality control, ensuring the accuracy and purity of your oligonucleotides. This approach also offers greater flexibility, enabling researchers to efficiently meet their specific needs while ensuring data integrity and cost-effectiveness. Q: Can LGC Biosearch Technologies help scale up manufacturing if we develop the initial oligos ourselves?
Yes. Biosearch Technologies can assist in scaling up manufacturing for oligos initially synthesised in your lab. Our experts can help streamline the transition from small-scale production to larger volumes while meeting GMP guidelines.
Q: How much training is required to operate your MerMade oligo instrument?
MerMade oligo instruments typically require a standard training period of just three to four days. View the full range of our MerMade instruments here.
Q: What kind of support is available to customers who have purchased a MerMade oligo synthesizer from your company?
 We offer comprehensive support for customers who have purchased MerMade oligo synthesizers, including, training during installation, online support, and assistance with protocol suggestions. Post-warranty, we also offer various service contract options to suit your needs.
We offer comprehensive support for customers who have purchased MerMade oligo synthesizers, including, training during installation, online support, and assistance with protocol suggestions. Post-warranty, we also offer various service contract options to suit your needs. Q: Is there a need to clean the oligo synthesizer between two synthesis rounds, and if so, how time-consuming is the process?
There is generally no need for cleaning between runs. If reagent replenishment is necessary, it takes approximately 20 minutes. Loading new columns may take 2 to 10 minutes, depending on the model. Setting up a new run typically takes 2 to 5 minutes.
Q: Are there limitations on the length of oligos that your machines can synthesise?
Our machines can produce oligos longer than 100 nucleotides. Any limit on the length of an oligo is determined by chemistry rather than the instrument. Different solid supports are optimal for different oligo lengths. We can support selection of an appropriate solid support for your oligo. You can get started with this online decision tool, created in collaboration with SelectScience.
Q: How can a 6-oligo machine produce 6 oligos and a 96-oligo machine produce 96 oligos in the same amount of time?
Oligo synthesis on all MerMade machines occurs in parallel, so it takes around the same amount of time to make 1 or 192 oligos. The timing is calculated based on cycle time and instrument movement. Explore the full range of oligo synthesizers from Biosearch Technologies.
Q: I need to synthesise oligos ranging from 20 to 60 nucleotides. Which oligo synthesizer would you recommend?
All MerMade synthesizers can produce oligos in the 20-60 nucleotide range. The choice of instrument depends on how many oligos you want to make per day and the synthesis scale.
Compare our MerMade synthesizers that support production at a range of scales, flexibility and throughputs.
| Oligo synthesizer | Throughput | Columns format | Max amidite positions (standard) | Columns format | Scale | Benefit |
| MerMade 4 | low | 4 | 10 | 20mer primers in 3.5 hours on each column | 50 nmol to 5 μmol | Each of the four columns can be assigned a different protocol to synthesise oligos of different quality and yield within the same run. |
| MerMade 6 | medium | 6 | 10, 16, 20 and 24 | 20mer primers in 3.5 hours on each column | 50 nmol to 200 μmol | Capable of making a combination of standard, degenerate and modified oligos in the same run. Unmatched flexibility, upgrade ability, dependability, efficiency and ease of use. |
| MerMade 12 | medium | 12 | 10, 16, 20 and 24 | 20mer primers in 3.5 hours on each column | 50 nmol to 200 μmol | Ideal for labs requiring medium throughput with maximum flexibility. Capable of making a combination of standard, degenerate and modified oligos in the same run. |
| MerMade 48X | high | 4 plates of 12 columns | 20 or 24 | 20mer primers in 3.5 hours | 50 nmol to 5 μmol | A fast oligo synthesizer that is capable of producing high quality oligos – even those with extensive modifications. Allows for hot swapping plates as the synthesis is completed to facilitate a continuous, efficient operation. |
| MerMade 96E | high | 96 well format of columns | 12 | 20mer primers in 3.5 hours | 50 nmol to 1 μmol | Ideally suited for labs that need a fast synthesizer that can be configured for all types of chemistry and is capable of producing high-quality oligos. |
| MerMade 192E | high | 2x 96 well format of columns | 8 | 20mer primers in 3.5 hours | 50 nmol to 5 μmol | Produces a very high-quality product with very minimal reagent usage. Ideal for those who need a fast synthesizer that is capable of producing high-quality oligos that do not require extensive modifications. |
| MerMade 192X | high | 2x 96 well format of columns | 12-64 | 20mer primers in 3.5 hours | 50 nmol to 5 μmol | This oligo synthesizer adds even more flexibility, configurability and speed compared to models listed above. It can be configured to synthesise all types of chemistries and produces high-quality oligos. |
Explore the full range of oligo synthesizers from Biosearch Technologies.
Q: Which synthesizer is best for synthesising very long oligos?
You can select any of our synthesizers because they are all equipped to synthesise long oligos. However, the quality and length of the oligo, as well as fidelity, depend on factors such as reagent quality, reagent freshness, and adherence to proper chemistry techniques.
We have more than 1000 specialised products for oligo synthesis. Download the catalogue here
Q: Can you provide a price range for different oligo synthesizers?
The price range for our oligo synthesizers falls between $320,000 and $920,000.
Q: Which activator is better, ETT or DCI?
5-ethylthio-1H-tetrazole (ETT) is an effective activator due to its higher solubility properties and greater acidity. Alternatively, 4,5-dicyanoimidazole (DCI) is a less acidic but more nucleophilic activator, which can reduce coupling times. DCI is highly soluble in acetonitrile, allowing for higher effective concentrations of nucleoside phosphoramidites during solid-phase synthesis, resulting in lower phosphoramidite excess during coupling. Explore our range of activators
However, highly pure DCI must be used for oligonucleotide synthesis to prevent errant contaminant peaks in crude oligonucleotides. Additionally, DCI is the preferred choice for assembling oligonucleotides containing 2′-deoxy-2′-aminopyrimidines due to its greater efficiency. Please note that Biosearch Technologies does not currently sell DCI.
Explore the complete portfolio of synthesis reagents and instruments from Biosearch Technologies.
Q: Why is DCA recommended over TCA, and how does the percentage relate to reaction time?
DCA (Dichloroacetic acid) is preferred over TCA (Trichloroacetic acid) for detritylation because it is a milder acid, making it a suitable choice for longer oligonucleotides. It often yields better synthetic results compared to TCA. However, TCA, though harsher, can reduce the deblock cycle from 45 seconds to 35 seconds.
A 3% v/v DCA/DCM solution can improve the rate of detritylation with DCA. This solution is effectively 4.5% w/v and 1.5 times as concentrated as a 3% w/v TCA/DCM solution. When transitioning from TCA to DCA, doubling the deblock delivery is recommended to ensure thorough removal of DMT (dimethoxytrityl) groups, as incomplete removal can result in deletion mutations. With the increased DCA delivery, significant depurination issues have not been observed.
Q: Does using a 3:1 mixture of ammonium hydroxide and ethanol instead of just ammonium hydroxide for cleaving/deprotecting oligos require an increase in time or temperature?
Yes, it does. This condition is suitable for mild deprotection, such as standard RNA or long oligos for gene synthesis, to prevent depurination and deamination. The required adjustments may also depend on whether you're using regular or fast deprotecting amidites.
Q: What about the unpleasant smell and potential toxicity of the reagents used for oligo synthesis? Are they hazardous to work with?
Precautions are essential when handling all synthesis reagents due to their toxicity. It is crucial to work in a well-equipped laboratory with proper ventilation, PPE, flammable storage cabinets and arrangements for safe disposal of organic solvents. For comprehensive safety information, refer to the product safety data sheets.
Q: What could be the cause of randomly missing bases in the primer binding region or overhang after amplification of VL and VH fragments from a cDNA pool?
This indicates poor synthesis quality and suggests the need for a more precise primer design.
Q: I purchase primers from different companies and notice a wide variation in the yield for the same scale. What factors contribute to these differences?
The differences arise from synthesis quality, freshness of reagents, reagent quality and the cycling conditions used. Additional, yield can be impacted by using insufficient reagent amounts per coupling or not allowing for enough time during each step. 

Q: How do I purify long oligos?
To start, it's advised to use DMT when purifying oligonucleotides that exceed 70 mer in length. HPLC and PAGE are the preferred purification techniques.
Reverse-phase HPLC is ideal, but it might compromise both purity and yield. On the other hand, denaturing PAGE offers high purity and ensures a significant proportion of full-length oligonucleotides, though its yield can be less than that of HPLC.
Q: Why do you recommend drying down the oligo before purification?
Drying down the oligo before purification is a practical step that helps remove solvents, adjust concentration, improve compatibility with purification methods, reduce contaminants and enhance the stability and handling of the oligo. These benefits contribute to the overall success of the oligo synthesis and purification process. After deprotection, it is advised to dry ammonia or AMA, then resuspend in water or your preferred buffer before purification. Evaporating ammonia prevents altering the sample concentration.
Q: What's the optimal oligo length for HPLC purification?
Anion exchange HPLC works best for purifying oligonucleotides up to around 40 bases in length. For large-scale synthesis, reverse-phase HPLC is suitable for oligonucleotides under 50 bases. While it's possible to purify longer oligonucleotides using this method, both purity and yield might suffer. Generally, HPLC purification yields better results for oligos fewer than 50 bases long.
Q: How should I store my oligos and how long do they remain stable?
Oligos can be dehydrated and stored at -20°C for up to 12 months. However, for extended storage, it's advisable to conduct a pre-experimental assessment. To improve their shelf life, minimise repeated freeze-thaw cycles, and prevent exposure to light, acidic conditions and nucleases.
Q: Can synthetic oligos interact with plastics, causing adherence in varying ways?
Studies show polyethylene terephthalate (PET) and polystyrene (PS) had the highest efficiency for the adsorption of linear DNA. Metal ions (Na+, Mg2+ and Ca2+) can promote the adsorption of single-stranded DNA (ssDNA) onto microplastics. Moreover, another study demonstrated that using amine-modified oligo probes resulted in better immobilisation efficiency on plastic surfaces compared to unmodified oligos.
WEBINAR: Listen to nucleic acid synthesis expert Lina Borozdina deliver a complete webinar masterclass on oligo synthesis.



