Originally published : Wed, March 25, 2015 @ 8:02 PM
Updated : Fri, November 13, 2015 @ 10:40 PM
In the setup of a qPCR experiment, one of the first decisions to make is which detection method to use. This generally boils down to two common options:
- DNA-binding Dyes
- Dual-labeled Probes
The DNA binding dyes, also known as intercalating dyes, are often referred to as SYBR® Green assays due to the popularity of this particular detection dye’s trade name. On the other hand, the most common probe-based format is the dual-labeled probe (hydrolysis probe) positioned internally to the amplicon sequence, containing the desired fluorophore on one end and a quencher at the other.
When choosing between either a dye or probe-based detection method, many researchers often default to using intercalating dyes based solely upon the notion that they cost less. However, when you really crunch the numbers, you might be surprised to learn that, depending on the experimental setup, SYBR-based systems are not always the most cost-effective. Therefore, it is important to thoroughly consider all aspects of an experimental design before generalizing the costs of each format. The bottom line is that in the long term, if 2 targets or more are multiplexed into a single reaction, reduced reagent costs make probe-based PCR the more cost-effective option.
Fast Food or Home-cooked Meal?
The dynamics of comparing costs of each detection method can be explained with the example of preparing a meal. What do you do for a meal if there is no food at home and you have a limited budget? Is your money best spent on a home-cooked meal or is it better to pick up fast food? Well, that depends on your needs.
When you’re eating alone, buying a single Value Meal from your local fast food restaurant is considerably cheaper than buying all the ingredients to prepare a similar meal at home. Spending a few bucks each day for a meal, one could feasibly do this for an extended period of time. However, if multiple people are involved, ordering a meal for each person gets less economical. Although ordering fast food or take out is relatively inexpensive for a single person, costs multiply as more people are involved, which makes this option unsustainable in the long term.
Instead of ordering fast food or take out, each meal could be cooked at home, which requires buying a number of ingredients. While money can be saved in the long term, the initial cost for all the ingredients is more than the single fast food meal. For example, ingredients like condiments or spices used to flavor your meal can cost a lot upfront, but you can get days or months of use out of it. Ultimately, the cost to cook the meal at home is actually often less than ordering the same meal, and the decision to order or cook food comes down to whether the short-term or long-term cost is more important. Unlike ordering food though, the benefits to cooking each meal increase as more people are involved. Despite any extra money spent on additional ingredients to feed more people, the cost to cook the entire meal increases by only a marginal amount, and the cost per person actually decreases! As the number of people you are feeding increases, it is clear that grocery shopping increasingly becomes the more cost effective approach.
Breaking Down the Costs of your Experiment.
We know that your qPCR experiments aren’t food, but we can use the above analogy to illustrate some important points: let’s say that the reaction is equivalent to the meal (SYBR-based assay = fast food meal, Probe-based assay = home-cooked meal), the number of people is equivalent to the degree of multiplex, and the cost per target per reaction is equivalent to the cost per person for a meal. Consider the costs and benefits of using both SYBR and a probe to detect your target(s). If you only plan on running a handful of assays against a single target, ordering a SYBR-containing master mix and some primers will likely prove to be relatively inexpensive (although, even in this scenario, one should always consider whether their experimental design or target(s) require the extra level of specificity provided by a probe!). If you want to amplify and detect multiple different targets at a time though, you need to either use a probe-based chemistry or run each target in a separate reaction. While it seems feasible to simply buy some additional primer sets, SYBR assays are incompatible with multiplexing because multiple targets can’t all be detected in a single reaction. Since each target has to be run in a separate reaction, more master mix, which is the most expensive consumable in the experiment, ends up being used. This leads to a multiplication of the costs with each additional target. If each target is detected by a different fluorophore, as in probe-based qPCR, multiple targets can be amplified in a single reaction, which limits the amount of master mix required.
To demonstrate this, the cost per reaction was calculated using Biosearch oligos and master mixes from 10 well-known manufacturers (typically the 5 mL size) and averaged. The cost of reagents for a single reaction is $0.56 if SYBR is used and $0.82 if a probe is used. However, if an additional target is added to the experiment, a SYBR assay would double in cost to $1.13 per reaction because of the requirement for more master mix, while a probe-based assay only marginally increases in cost to $0.89 because both targets can be detected in one reaction. This information is illustrated in the infographic below, where the left side (purple background) corresponds to probe-based assays and the right side (green background) corresponds to intercalating dye (SYBR) based assays.
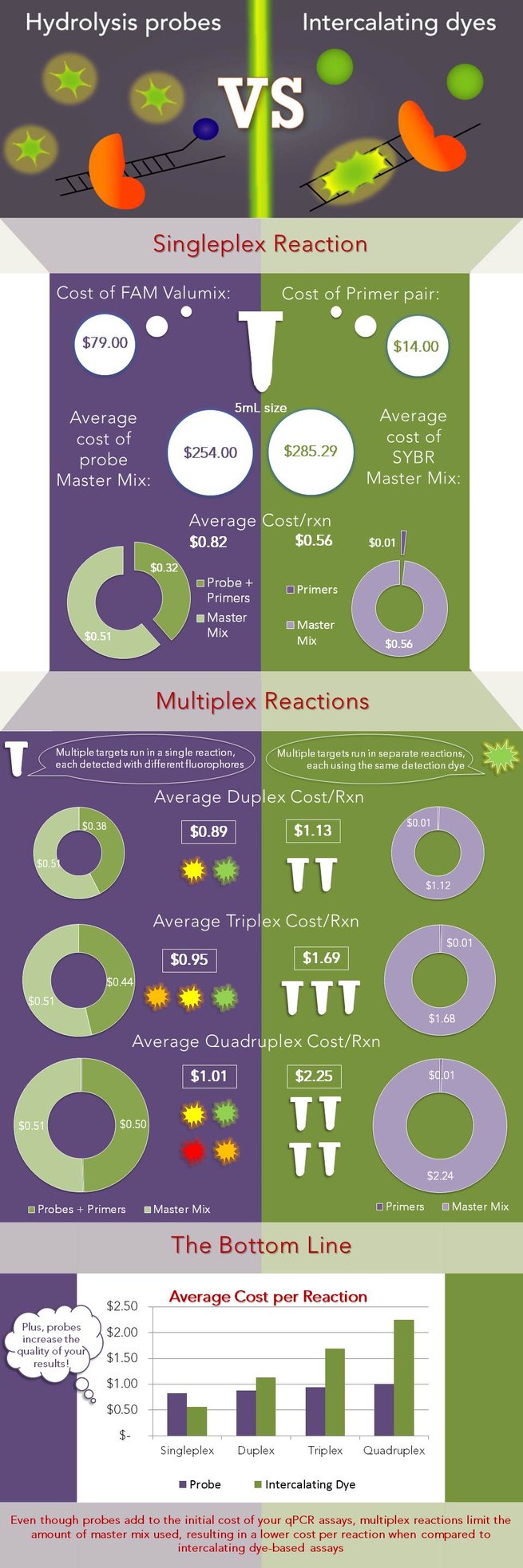
The average cost per reaction is shown for both SYBR and probe-based assays. The cost per reaction was calculated across a number of master mix manufacturers, by adding the cost per reaction of the master mix to that of the oligos, and averaged. The reaction size was assumed to be 20 μL with 300 nM of each primer and 100 nM probe. The final graph shows that as the number of targets increases (increasing the degree of multiplex of the reaction), SYBR-based reactions multiply in cost, while probe-based reactions have only a marginal increase in the cost/reaction.
While the cost savings for probe-based assays increase with the degree of multi-plex of the reaction, you don’t need to be running quadraplexes to reap the benefits that probes have to offer. Even if you’re only running two targets, such as the target of interest and a reference target (as is common practice), it is almost always more cost-effective to buy two probes than to buy double the volume of master mix. Assuming a 20 µL reaction volume and a 100 nM probe concentration, you can run 500 reactions for every nanomole of probe delivered. (Hint: use the Reaction Estimator in the qPCR Design Lab toolbox to determine how many reactions can be run with your oligos). Thus, a CAL Fluor® Probe adds $120 to the cost of the experiment and can be used for up to 2500 reactions. Whereas, running 2500 additional SYBR based reactions, in comparison, could add anywhere between $655 to $1712 to the cost of the experiment, depending on the master mix used! Using one manufacturer as an example, an immediate savings of almost 50% can be achieved if the user runs a probe-based duplex reaction across only 40 samples. The sample count at which using probes becomes cheaper than using intercalating dyes will vary with different master mix distributors; for some companies, each master mix (either SYBR-containing or not) costs the same, but for several others, the SYBR master mix is considerably more expensive, further demonstrating that SYBR-based assays aren’t always the most cost-effective.
Data Quality
Additionally, the quality of the data should not be overlooked when considering cost. Going back to the food analogy: when you cook a meal at home, you know exactly what ingredients are in it and can be sure that what you are eating is healthy, if that is what you desire. By ordering a meal, you sacrifice knowing exactly what ingredients were used to prepare it, and if you’re not careful when ordering might get a meal containing undesired ingredients. Similarly, when a probe is used in your qPCR assay, you can have more confidence in the results you obtain. Probes add another layer of specificity and security to the reported assay signal, while intercalating dyes have the potential to indiscriminately report signal from spurious amplification products (like primer dimers), resulting in potentially misleading results.
The Bottom Line
Like our fast food vs. home-cooked meal analogy above, the cost breakdown of a qPCR assay favors a probe-based detection method as long as a large number of samples are being analyzed. Considering that many experimental designs require the normalization of the gene of interest to a reference gene, and that larger sample sizes increase the power of a statistical test1,2, it is pretty likely that for any given experiment a probe-based detection method saves the user money. Not only does this reaction setup require fewer consumables, but there is less technical variability involved, since multiplexing allows you to normalize within the same well on the same aliquot of a sample! Further, with the added layer of specificity, it yields more statistically sound, quality data. Although using SYBR does prove to be more cost efficient when fewer assays are run (similar to a single person purchasing a fast food value meal), it is important that the end user consider the number of targets and samples that they will be analyzing over time, prior to determining which approach will actually be best for their budget. If cost is a limiting factor when planning your experiments, don’t be fooled by the seemingly low cost of running intercalating dye based assays!
References
- Statistical aspects of quantitative real-time PCR experiment design. Kitchen RR, Kubista M, & Tichopad A. Methods. 2010 Jan; 50: 231–236.
- Why the need for qPCR publication guidelines?--The case for MIQE. Bustin SA. Methods. 2010 Jan; 50: 217–226.

