Originally published : Mon, August 21, 2023 @ 2:57 PM
Labelling, linking or modifying biomolecules used to be a laborious process, requiring customised complex synthetic pathways that yield little product. However, click chemistry has been a major development in rapidly and efficiently constructing biochemical structures. This Nobel Prize-winning approach enables users to easily connect two molecules using readily available reagents under simple, highly selective, and efficient reaction conditions.1,2
One of the most widely-used click reactions is Huisgen’s 1,3-dipolar cycloaddition in the presence of copper(I), commonly called the Cu- alkyne–azide cycloaddition (CuAAC). It is a two-step reaction between azides and terminal alkynes, catalysed by copper(I), to form 1,2,3-triazole (figure 1).3,4
The high efficiency of click chemistry has made it a versatile tool that can be applied across multiple fields. This reaction has been increasingly used in biology as it is easy to incorporate azide and alkyne groups into large bio-organic molecules. These groups are compatible with biological molecules, as they do not react with the functional groups found in nucleic acids, proteins and lipids.3
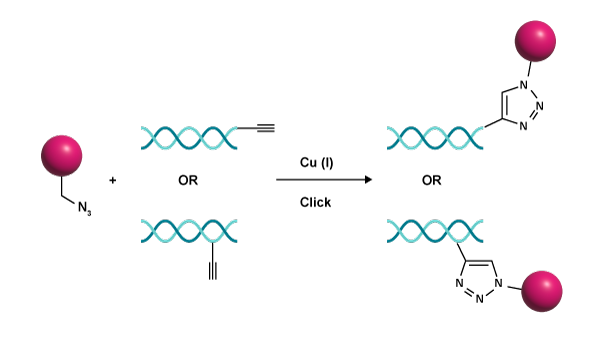 |
| Figure 1: The Copper(I) alkyne-azide cycloaddition (CuAAC) reaction became widely used due to its simplicity and versatility in bonding two molecules together. |
Modification of biomolecules using click chemistry
Click chemistry offers a quick, simple and streamlined approach to joining organic molecules without the need for heating, high pressure, and complex purification methods (there are no condensation side-products of click reactions). It is a reliable and versatile technology ideal for applications in bioconjugation, probe-labelling, screening assays, and the development of pharmaceuticals. Here we will briefly examine how click chemistry can be used to modify biomolecules used in molecular biology techniques, particularly oligonucleotides, as well as proteins and antibodies.
Oligonucleotides
The CuAAC reaction quickly became a popular method for DNA click chemistry due to its exceptional efficiency. The technique is widely used to label DNA, RNA and locked nucleic acid (LNA) oligonucleotides, as well as to cyclise DNA, join oligonucleotides to peptide nucleic acids (PNA), synthesise DNA catenanes and create analogues of DNA with modified nucleobases and backbones.4
Oligonucleotide labelling
Click DNA labelling is commonly used to post-synthetically conjugate alkyne-labelled DNA to azide-functionalised reporter groups such as fluorescent dyes, sugars and peptides. The reaction is high yielding and the reactive components remain stable in aqueous buffers.3,4 Additionally, the stability of the alkyne group during solid-phase synthesis enables the post-synthetic labelling of oligonucleotides. This is particularly useful when attaching dyes to oligonucleotides that cannot be incorporated during synthesis because they may degrade under the strong basic conditions necessary for DNA and RNA cleavage and deprotection.3 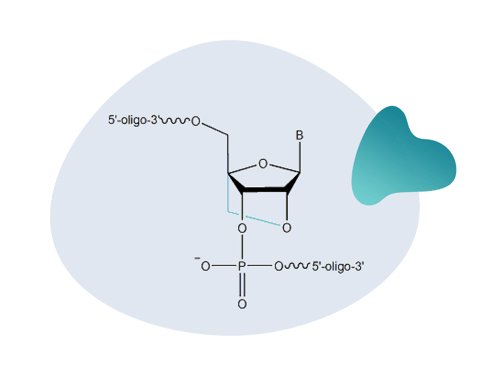 Fluorescence-labelled oligonucleotides have applications as probes and primers for molecular assays such as fluorescent in situ hybridisation (FISH), quantitative PCR, sequencing and live cell imaging, which are routinely used in molecular diagnostics and drug development.3,4 Furthermore, clickable LNA and DNA oligos can be linked to carbohydrates, lipids, cofactors, cell-penetrating peptides and metal complexes, which can be used to develop biosensors, therapeutics, and nanoconstructs.4
Fluorescence-labelled oligonucleotides have applications as probes and primers for molecular assays such as fluorescent in situ hybridisation (FISH), quantitative PCR, sequencing and live cell imaging, which are routinely used in molecular diagnostics and drug development.3,4 Furthermore, clickable LNA and DNA oligos can be linked to carbohydrates, lipids, cofactors, cell-penetrating peptides and metal complexes, which can be used to develop biosensors, therapeutics, and nanoconstructs.4
Oligonucleotide immobilisation
Click chemistry can efficiently immobilise oligonucleotides onto hard or soft substrates, such as glass, silicon and polymers. Typically, azide-functionalised DNA, PNA or modified nucleic acids are linked to alkyne end-functional diblock copolymers.4 Such methods have been used to construct microarrays, fluorescence-microscopy slides and DNA-immobilised beads for diagnostics and biomedical research.4,5
Other nucleic acid assemblies
Several studies have demonstrated the use of click chemistry in a variety of nucleic acid assemblies, including ligation, circular constructs and catenanes. Click ligation has been employed to generate stable 5′–5′ and 5′–3′ strand ligation, the synthesis of cyclic DNA catenanes (synthetic mimics of mini-plasmids), mini-DNA duplexes, and duplexes that are covalently cross-linked across the nucleobases.3 Click-ligated small-cyclic DNA and mini-duplexes are being explored in drug-DNA binding analysis and as potential therapeutics because of their higher resistance to nucleases, high thermal stability and better cell penetration.3,4
Antibodies
The adaptability and efficiency of click chemistry allow its use in bioconjugating antibodies used for biomedical research, molecular diagnostics and potential therapeutics. Site-specific labelling of antibodies with fluorescent dyes, peptides, DNA and small molecules can be achieved by introducing clickable handles at specific sites, for example, at lysine or glutamine residues.6 These strategies have been successfully employed in constructing antibody-drug conjugates and bispecific antibodies for cancer therapeutics.7,8
Proteomics and ligands
Click chemistry can be used to identify and quantify proteins through selective labelling and enrichment. It’s commonly used to map small molecule-protein interactions and various post-translational modifications. Proteins of interest are first labelled by treating tissue, cells or lysates with a tag containing a click handle (either azide or alkyne). Then, they are exposed to click reaction conditions with a complementary click functional group linked to a tag for protein enrichment, such as biotin. This approach is widely used in various areas of proteomics research.9
Click chemistry reactions are also used to develop selective ligands for receptor activation studies and fragment-based enzyme inhibitors.2
Applications of click chemistry in research, therapeutics and diagnostics
The modularity, scalability and biocompatibility of click chemistry has quickly garnered interest in the field of medical research, drug development and diagnostics. It has become a powerful tool for bioconjugation and high-throughput screening, opening up new possibilities and significantly expanding the molecular toolbox for understanding complex biological systems.
Below we list for you some examples of how click chemistry can be applied across biology and biotechnology.
Copper-free click chemistry with bicyclononynes
An alternative click reaction that avoids the need for a catalyst uses a cyclic alkyne with strained geometry to lower the activation energy (figure 2). The cycloaddition between a cyclooctyne and an azide opens many opportunities where copper might compromise cell function or degrade oligonucleotides.
The Click-easy™ products from LGC Biosearch Technologies use a bicyclononyne (BCN) scaffold, which offers a balance of reactivity and lipophilicity. These allow oligonucleotides to be efficiently labelled with the alkyne, ready for a click reaction with an azide of choice. BCN moieties can be incorporated during oligo synthesis using the phosphoramidite form or attached to other biomolecules using esters.
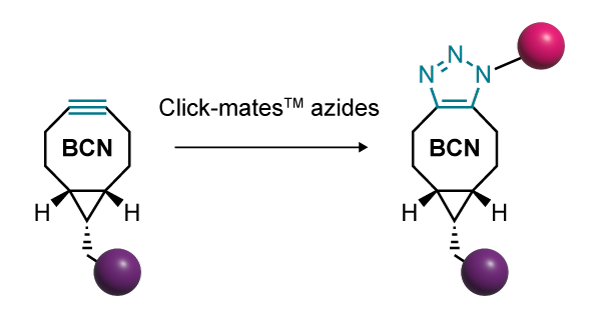 |
| Figure 2. A bicyclononyne scaffold is sufficiently strained that the click reaction can proceed without the need for a potentially toxic catalyst |
In research published in ACS Nano, Marth et al. used 5′-Click-easy BCN CEP II modified oligonucleotides that could click easily to modified proteins containing an azido-phenylalanine residue.10 This allowed the researchers to precisely attach a DNA strand, giving single-molecule control that could be used for fine-tuning protein assemblies on DNA origami tiles. In 2017, Arcuri et al. used click chemistry to understand how to improve a new typhoid vaccine designed to work for infants.11 This was a glycoconjugate vaccine, where the antigen is conjugated to a carrier protein to enhance the immune memory response. Using Click-easy BCN N-hydroxysuccinimide Ester I, the team tested how the vaccine’s immunogenicity was influenced by different linkers and other chemical variables. The authors of the study suggested that the approach they demonstrated could be used to optimise the efficacy of other conjugate vaccines.
In 2017, Arcuri et al. used click chemistry to understand how to improve a new typhoid vaccine designed to work for infants.11 This was a glycoconjugate vaccine, where the antigen is conjugated to a carrier protein to enhance the immune memory response. Using Click-easy BCN N-hydroxysuccinimide Ester I, the team tested how the vaccine’s immunogenicity was influenced by different linkers and other chemical variables. The authors of the study suggested that the approach they demonstrated could be used to optimise the efficacy of other conjugate vaccines.
Screening of therapeutic molecules using synthetic organisms
In a study aimed at engineering a potentially safer and more efficacious version of recombinant human cytokine interleukin-2 (rhIL-2) for cancer therapy, researchers generated a library of rhIL-2 variants with azide-containing residues using a semi-synthetic, six-letter DNA code in an engineered organism.12 The expanded genetic code within the insert fragments of the IL-2 and anticodon-containing pylT tRNA expression vector were chemically synthesised using orthogonal dTPT3 and dNaM phosphoramidites.
The team then applied click chemistry to selectively PEGylate these variants, allowing promising candidates to be identified. This would have been difficult or impossible to produce using traditional methods such as site-directed mutagenesis or lysine-mediated chemical coupling.
Diagnostic cyclic imaging using click-labelled antibodies
Click chemistry was utilised in a diagnostic assay to identify biomarkers for subtypes of salivary gland tumours.13 FAST cyclic imaging method was applied on single-cell homogenates and fine needle aspiration samples, where the cells were repeatedly stained with fluorescently labelled antibodies, quenching the fluorescence after each image acquisition cycle. This was facilitated by designing custom linkers containing a trans-cyclooctene (TCO) on specific sites of an antibody of interest, to which an IF tetrazine (Tz)-conjugated black hole quencher (BHQ) is clicked during each quenching step.13
The same technique was applied by another group from the same research laboratory to design affordable, rapid point-of-care diagnostics for oncology.14 Here, cells from the tumour specimen were attached to glass slides and then subjected to FAST imaging using Tz/TCO click reaction, supported by the quenching power of BHQ.
Click-chemistry for the preparation of DNA microarrays
Frydrych-Tomczak et al. previously demonstrated that the CuAAC reaction could be used to prepare DNA microarrays through oligonucleotide immobilisation on glass which is surface-functionalised using 3-azidopropyltrimethoxysilane (AzPTMS).15 In a recent study, they analysed the binding efficiency of different concentrations of silane layers for preparing DNA microarrays.16 The reactivity of azido functional groups towards 5′ alkynyl-terminated oligonucleotides and the stability of the bonding upon washing the slides with imprinted probes was evaluated using oligonucleotides labelled with a fluorescent tag (fluorophore CPG with Quasar 670) at their 3′ ends.
 Metal ion screening
Metal ion screening
Wu et al. used the CuAAC reaction to develop a fluorescence assay for detecting Cu2+.17 The assay employs a cascade signal amplification that relies on the presence of Cu2+ to trigger a click reaction between two modified DNA sequences. This creates an autocatalytic DNAzyme that can cleave the bond between a fluorophore and a quencher, producing a detectable signal. The assay is ultrasensitive, detecting as low as 2nM of Cu2+.
Click chemistry compatible reagents from Biosearch Technologies
The recent advances in the field of click chemistry have made a significant impact on many areas of biological research and bioengineering. One notable area of progress is oligonucleotide click chemistry, which has seen the development of several modifications and protocols. Its use in developing molecular diagnostics and biopharmaceuticals has the potential to transform patient outcomes and methods.
Unlock the power of research innovation with a wide range of click chemistry compatible reagents from Biosearch Technologies, plus other products that are often used in combination with click chemistry applications, such as fluorophores and quenchers.

