Originally published : Tue, June 28, 2011 @ 3:33 PM
Updated : Mon, September 19, 2022 @ 2:10 PM
A "Referral from the Doctor" Blog Article-
Fluorescent probes are used in biochemical assays to monitor specific events such as binding, cleavage or conformational changes. Dual-labeled probes with a fluorophore and a quencher have many applications in genetic analysis. The efficiency of fluorescence quenching is very distance dependent – if the reporter fluorophore and quencher are far apart, there is fluorescence; if the reporter and quencher are close together in space, fluorescence is suppressed. Typically, the reporter and quencher are placed at specific sites in an oligonucleotide such that a change in their distance will produce a maximal change in fluorescence and effectively signal the event being monitored (often hybridization or nuclease activity). The oligonucleotide acts as a flexible tether linking the fluorescent reporter and quencher. Below, we present fluorescence quenching mechanisms for dual-labeled oligonucleotides in genetic analysis.
 I. Static Quenching (also known as contact quenching):
I. Static Quenching (also known as contact quenching):
Static quenching involves physical interaction between a reporter and quencher dye resulting in a new, non-fluorescent species called an intramolecular dimer. The resulting dimer is a ground-state complex which has its own distinct features, such as its absorption spectrum. Static quenching efficiency depends on the affinity of the reporter and quencher for each other.
II. FRET (Förster Resonance Energy Transfer) Quenching:
 |
 |
FRET is a through-space mechanism in which energy from the reporter dye is transferred to the quencher without absorption or emission of light.
Efficient FRET requires:
a) Proximity: the donor and acceptor molecules must be close to each other (approx. 10 – 100 Å). FRET is extremely distance dependent.
b) Spectral overlap: the absorption spectrum of the acceptor must overlap with the emission spectrum of the donor.
c) Relative donor-quencher orientation: in most assays with fluorescent probes, it is assumed that the relative orientation of the dyes is random.
Key equation for FRET: 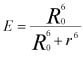
Where E is the efficiency of FRET energy transfer, R0 is the Förster distance which is the donor-acceptor distance at which energy transfer is 50%, and r is the distance between the donor and acceptor.
These mechanisms may operate independent or simultaneously. For more information on fluorescence quenching mechanisms, please visit our website at: http://www.biosearchtech.com/support/applications/quenching-mechanisms-in-probes.aspx
Written by: Mary K. Johansson, Ph.D., and Christina Ferrell, Ph.D.
References:
Efficiencies of fluorescence resonance energy transfer and contact-mediated quenching in oligonucleotide probes. Salvatore A. E. Marras, Fred Russell Kramer and Sanjay Tyagi. Nucleic Acids Research, 2002, 30, e122.
Intramolecular Dimers: A New Strategy to Fluorescence Quenching in Dual-Labeled Oligonucleotide Probes. Mary Katherine Johansson, Henk Fidder, Daren Dick and Ronald M. Cook. J. Am. Chem. Soc. 2002, 124, 6950.
Choosing Reporter-Quencher Pairs for Efficient Quenching Through Formation of Intramolecular Dimers. Johansson, M.K. Methods in Molecular Biology, v. 335; V.V. Didenko, Ed; Humana Press: Totowa, NJ, 2006; pp 17-29.
Intramolecular Dimers: A New Design Strategy for Fluorescence-Quenched Probes. Johansson, M.K.; Cook, R.M. Chem.-Eur. J. 2003, 9, 3466.

